Exploring the Reprogramming Potential of Fetal Sources of Induced Pluripotent Stem Cells and their Application in Regenerative Medicine
Caterina Pipino1* and Sayandip Mukherjee2
1Department of Medical, Oral and Biotechnological Sciences, “G. D’Annunzio” University, Italy
2Department of Biological Sciences, Bangalore University, India
*Address for Correspondence: Caterina Pipino, Department of Medical, Oral and Biotechnological Sciences, “G. d’Annunzio” University, 66100 Chieti, Italy, Email: c.pipino@unich.it
Submitted: 24 June 2016; Approved: 28 December 2016; Published: 29 December 2016
Citation this article: Pipino C, Mukherjee S. Exploring the Reprogramming Potential of Fetal Sources of Induced Pluripotent Stem Cells and their Application in Regenerative Medicine. Int J Stem Cell Res. 2016;1(1): 001-006.
Copyright: © 2016 Pipino C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Induced Pluripotent Stem Cells; Fetal Cells; Regenerative Medicine
Download Fulltext PDF
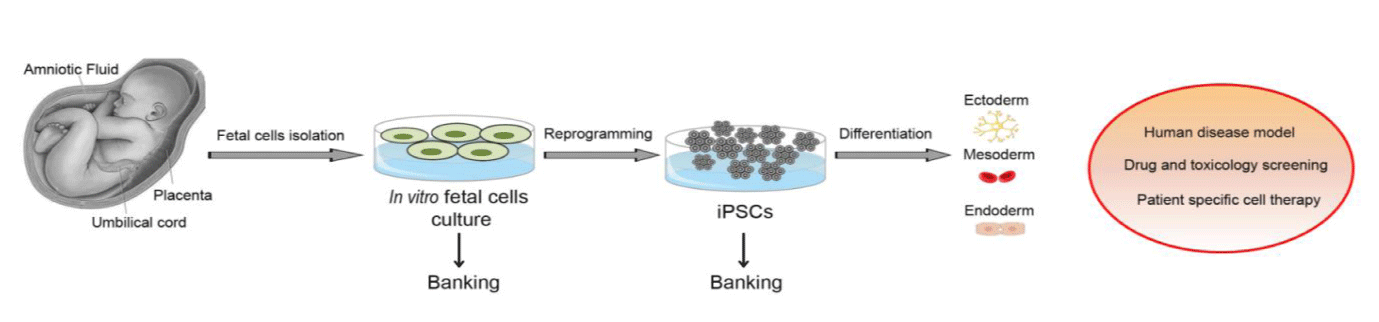
Reprogramming to pluripotency is possible from adult cells of different tissues and species through the ectopic expression of defined factors. The generated induced Pluripotent Stem Cells (iPSCs) are relevant for various purposes, including disease modeling, drug or toxicity screening and autologous cell therapy. Over the last few years, increased efforts are being made to improve the reprogramming techniques, the efficiency and quality of the generated iPSCs, as well as to identify the best cell source to be reprogrammed. Cells derived from fetal tissues, such as amniotic fluid, placenta and umbilical cord, offer distinct advantages in terms of reprogramming compared to adult somatic cells. Importantly, fetal cells are more primitive, easily achievable in sufficient numbers and are devoid of any ethical concern. They show great plasticity, high proliferation rate, low immunogenity and absence of teratoma formation. Therefore, they can be reprogrammed much faster and more efficiently than adult cells. Here, we provide a comprehensive overview of the advantages of reprogramming fetal sources in comparison to other commonly used cell types.
Introduction
The technology to reprogram adult human terminally differentiated somatic cells to a pluripotent stem cell state has opened up unprecedented avenues in the realms of disease modeling, high-throughput screening of novel drug candidates, cell therapy and personalized medicine. Induced Pluripotent Stem Cells (IPSCs) are obtained through a simple genetic manipulation of adult somatic cells by the over-expression of a defined set of transcription factors which confer upon them a pluripotent state, similar to Embryonic Stem Cells (ESC). Although iPSCs sharemany common features with ES cells, a number of research groups have reported that human iPSCs differ notably from human ES cells in terms of gene expression, chromatin methylation patterns, proliferative capacity, and most significantly in the susceptibility of the differentiated progeny to senescence and apoptosis [1–5].
The original reprogramming cocktail of Yamanaka and Takahashi included the transcriptional regulators, namely OCT3/4, SOX2, KLF4, and c-MYC, which were responsible for both induction as well as maintenance of the pluripotent stem cell state [6,7]. Subsequently it was demonstrated by various other groups that while SOX2 and OCT3/4 are essential for reprogramming, KLF-4 and c-MYC are dispensable and can be replaced by LIN-28 and NANOG without sacrificing the efficiency [8]. Further evidences regarding the mechanistic plurality of reprogramming were obtained by the employment of a host of small molecules, mostly epigenetic modifiers, which could effectively enhance the reprogramming frequency as well as fine tune the epigenetic signature to match those of ES cells [9–11]. While the quest for the minimally non-invasive (in a genomic way) reprogramming cocktail continued, Park and colleagues generated panel of patient- and disease-specific iPSCs from individuals with monogenic disorders [12]. They provided proof-of-principle vis-à-vis the employment of these patient-derived iPSCs for cellular modeling by directed differentiation of the iPSCs into various cellular lineages of choice and by successfully demonstrating that these cells possess the same genetic characteristics of the donors thereby recapitulating the disease phenotype. Generating iPSCs as in vitro disease model will be useful not only for drug screening but also for elucidating mechanisms of disease pathogenesis.
The iPSC technology overcomes two important obstacles associated with ES cells: first, ethical concerns surrounding the use of human embryos, and second, clinical concern regarding immune rejection post-transplantation of ESC-derived cells. The first concern is laid to rest by the inherent simplicity of iPSC technology in which a range of readily accessible somatic tissue sources like peripheral blood cells, skin fibroblasts and keratinocytes are reprogrammed to ES-like cells and embryos are not required. The second concern is also addressed as iPSCs support the generation of patient-specific autologous pluripotent cell-derived progenitors and precursors that are expected to match immunophenotypically thereby eliminating the risks of immune rejection during transplantation as well as offering an unique scope for in vitro disease modeling. Although they represent an excellent in vitro source and have provided proof-of-principle for tissue-regeneration in animal models [13,14] a few hurdles remain before the iPSC-derived cells can be adopted for widespread clinical application. The transplantation of autologous iPSC-derived retinal epithelium targeting macular degeneration has been conducted on a seventy-year old woman in Japan in September 2014 as the world’s first phase I clinical trial with iPSCs. Regardless of this clinical trial, US FDA harbours major concerns regarding the application of iPSCs in clinical trials. The two main concerns involve the reduction of genomic footprint of the reprogramming vector in the target cells, and ensuring the transplantation of a pure population of target cell progenitors derived from gene-corrected autologous iPSCs. While intense research is being pursued to develop reprogramming protocols that would allow large-scale production of “vector-free” iPSCs, eliminating the risk of developing teratoma from even a single pluripotent cell present in a population of differentiated cells is a significant challenge [15]. In light of the above, we aim to provide a brief overview of the alternate somatic sources for direct reprogramming.
Extra-embryonic cells
One of the principal aims in the field of iPSC generation is to identify the best cell source to reprogram. Pluripotent stem cells have been generated from cells of different somatic tissues such as fibroblasts, keratinocytes, blood, stomach and liver cells, neurons and hepatocytes [7,16–20]. To obtain adult somatic cells for reprogramming, invasive methods are necessary. Furthermore, in the case of humans it is difficult to obtain a biopsy from some tissues and, when feasible, adult cells are difficult to expand in culture in sufficient number, thus limiting the donor cell source for the reprogramming process. Additionally, accumulated mutations in adult cells and possibilities of insertional mutagenesis due to the use of retroviral and lent lentiviral vectors to deliver the transcription factors may affect the success rate of reprogramming process [21].
In the last few years, it has been demonstrated that stem cells from extra-embryonic tissues, such as placenta, amniotic fluid and umbilical cord hold advantages in reprogramming when compared with adult cells. Indeed, they possess an intermediate phenotype between embryonic (pluripotent) and adult (multipotent) cells, being therefore easier to reprogram. Stem cells from placenta and amniotic fluid can be derived at first- and mid-trimester from samples that once used for genetic tests are normally discarded or, as also in the case of umbilical cord, at the time of delivery. Fetal cells are younger and less epigenetically modified than adult cells, hence genetically more stable carrying fewer mutations [22–25].
With the goal of clinical application the source of donor tissue can be allogenic or autologous. The latter is preferred as it is not rejected by the recipient’s immune system and does not require a regime of immunosuppressant drugs. However, a limitation is the ex vivo expansion capacity of adult cells in adequate numbers for transplantation. Furthermore, in the case of particular organs, primary cells cannot be expanded in vitro. For all these reasons, fetal cells represent a good cell source to largely expand in culture and to reprogram, thereby providing an optimal cell source for cell replacement therapy.
Amniotic Fluid-derived Cells
Human Amniotic Fluid (AF) obtained in the early second trimester of pregnancy contains different cell types and among them a population of mesenchymal stem cells. These cells express typical mesenchymal surface markers, such as CD90, CD73, CD105 and the pluripotency markers OCT-3/4, SOX-2 and SSEA-4. They are negative for surface markers commonly expressed by embryonic stem cells, such as SSEA-3 and Tra-1-81 and negative for the hematopoietic markers CD34 and CD45 [26,27]. In addition, a subpopulation termed amniotic fluid stem cells, representing 1% of the total population, can be isolated by the selection of cells expressing the stemness marker CD117 (c-KIT) [28]. Importantly, these cells possess the capacity to give rise to the tissues of the three germ layers. They can be expanded in culture for many passages maintaining the length of telomerase and a normal karyotype and do not form teratomas when transplanted into immunocompromised mice, thereby demonstrating potential for regenerative medicine [28].
Work by our groups and by others have demonstrated that Amniotic Fluid (AF) cells can be easily and efficiently reprogrammed through the ectopic expression of the Yamanaka’s factors [29–32]. The generated iPSCs were positive for the pluripotency markers OCT-4, SOX2, SSEA-4, NANOG, TRA-1-60, TRA-1-81 and showed a normal karyotype. In addition they were able to spontaneously differentiate through mesodermal, endodermal, and ectodermal lineages both in vitro (embryo bodies formation) and in vivo (teratomas). Fully reprogrammed iPSCs could be generated from AF cells within 10 days of transduction [29] compared to three to four weeks or more from adult somatic cells [33]. This is probably due to the multipotent state of AF cells that may accelerate the reprogramming course [32]. In addition, samples from AF can be easily obtained for genetic tests by routine amniocentesis. Moreover, AF cells are transcriptionally and epigenetically similar to ES cells and having accumulated less mutations than adult cells, may be more easily reprogrammed [30,31,34,35].
Of note, iPSCs derived from AF cells represent an ideal source of cells for in vitro modeling of congenital diseases. We demonstrated that iPSCs derived from AF cells of fetuses with trisomy 21 maintained the genetic characteristics of the respective parental cells, hence representing a human cellular model for the in-depth study of these disease mechanisms [29]. AF cells are autologous to the fetus and therefore the generated iPSCs may allow the use of patient-specific cell source for regenerative medicine, disease modeling and drug screening. Of note, iPSCs could be cryogenically stored to produce a cell bank for researchers to access [29].
Interestingly, amniotic fluid cells from the prenatal diagnosis of a β-thalassemia patient have been efficiently reprogrammed using the STEMCCA lentiviral vector based on Cre/LoxP technology, thus offering a new approach for studying β-thalassemia [36] and other genetic diseases [37,38]. Liu et al. have demonstrated that CD34+ AF cells can be reprogrammed after infection with lentiviral vector encoding only OCT4 giving rise to iPSCs that were similar to ES cells as confirmed both in vitro and in vivo [39]. Another recent work also demonstrated that the OCT4 is sufficient to reprogram human AF cells into iPSCs, thus indicating the generation of patient-specific iPSCs without the transgenic expression of oncogenes [40]. Notably, AF cells have also been reprogrammed by a non-integrating episomal/EBNA plasmid in chemically defined culture conditions [41]. More interesting, c-KIT+ AF cells from both first and second trimester of pregnancy could be reprogrammed without genetic manipulations, only with the addition of valproic acid in a specific medium in ES cell conditions [42,43]. The absence of vectors to reprogram these cells may allow the use of safe iPSCs for application in regenerative medicine.
Placenta-derived cells
Recently, the placenta has emerged as source of stem cells with great potential in regenerative medicine that offer advantages in terms of proliferation and plasticity when compared with adult cells and permit to overcome the ethical and safety concern inherent in ES cells [44]. Placenta is composed of a fetal part (amniotic and chorionic structures) and a maternal part (decidua), both characterized by the presence of different stem cells. Of relevance, the placenta contains a population of broadly multipotent stem cells that also show expression of ES cells markers (c-KIT, OCT4, SOX2, SSEA3, SSEA4, TRA-1-60 and TRA-1-81). These cells have a mesodermal phenotype, but are able to differentiate, under appropriate conditions, not only into mesenchymal lineages, but also into hepatocytes, vascular endothelial, pancreatic and neuronal cells [45,46]. Mesodermal cells may also be responsible, in vivo, for the immunomodulatory function of the placenta. Indeed they express low levels of HLA-ABC and no HLA-DR, indicating their immune-privileged status, and, therefore, MSCs from placenta could successfully engraft in neonatal swine, sheep and rats, without rejection [47]. It has been demonstrated that human amnion-derived cells (hADCs), obtained from placenta after delivery, could be rapidly and efficiently reprogrammed into iPSCs by the defined factors: OCT4/SOX2/NANOG [18]. Indeed hADCs may be more easily reprogrammed than fibroblast cells due to their endogenous expression of KLF4 and c-MYC, indicating their potential to be rapidly reprogrammed without oncogene activation. Importantly, previous studies have shown that a subpopulation of hADCs expressed pluripotency markers, suggesting that hADCs might contain pluripotent stem cells, which could be induced to differentiate into the cells of the three germ layers [28,48]. Moreover, human chorionic villi cells were reprogrammed by transduction of retroviruses which express OCT4, SOX2, KLF4 and c-MYC [49].
Easley et al. have shown that Human Amniotic Epithelial Cells (HAECs) from placenta can be reprogrammed easily, faster and more efficiently than adult and neonatal somatic fibroblasts [50]. Of note, through epigenetic studies they found a less hypomethylated status in hAECs compared to adult and neonatal fibroblasts. This may partly explain why hAECs can be reprogrammed rapidly and more efficiently than adult and neonatal fibroblasts. In addition the epithelial origin of hAECs may promote a rapidly and efficiently reprogramming [16]. Indeed, it was demonstrated through microarray analysis that a Mesenchymal to Epithelial Transition (MET) event occurs during the first step of the reprogramming procedure [51]. Therefore, by using AECs for reprogramming, the initial MET phase could be skipped from the classic reprogramming process and then colonies appear earlier than neonatal and adult fibroblast [50].
In addition, placentas are usually discarded after delivery and their use do not imply ethical concern. Moreover, the procedure for isolating hADCs from placenta is relatively easy, fast, and safe. Another great advantage for the reprogramming process is the large quantity of hADCs available from placenta. Therefore, the generation of iPSCs from hADCs could allow the generation of a bank with the aim of autologous cell-replacement therapy in the later life of the cell donors. For all the reason listed above, placenta cells represent an ideal cell source that can be efficiently reprogrammed.
Umbilical cord-derived cells
The umbilical cord, a tissue that is traditionally discarded upon birth, contains a source of stem cells that can be collected rapidly, efficiently, and non-invasively from newborns at the time of delivery. Stem cells of umbilical cord can be isolated from cord blood and Wharton’s jelly, the connective tissue surrounding the vessels of umbilical cord [52]. These cells possess the advantage to carry less mutations when compared with adult donor cells.
Umbilical cord blood is a rich source of hematopoietic stem cells currently used in cell therapy transplantation with lower immunological reactivity and lower risk of graft-versus-host disease compare to those derived from adult bone marrow [53]. CD33+ hematopoietic stem cells from cord blood have been efficiently reprogrammed in two weeks time by retroviral transduction with OCT-4 and SOX2 only. Keratinocytes or fibroblasts were not able to generate iPSCs using the two factors only [54]. A population of MSCs is present in the cord blood but with low frequency and not in every samples collected [55]. Otherwise, MSCs from the Wharton’s jelly can be isolated in large number and are able to differentiate into adipogenic, osteogenic, myogenic, and chondrogenic lineages [56]. Moreover, Wharton’s jelly MSCs can also be induced to differentiate into dopaminergic neurons [57]. Pluripotent stem cells can be generated from MSCs of Wharton jelly by means of retroviral vector encoding the 4 Yamanaka factors on Matrigel without feeders [58]. The generated iPSCs showed the morphology of human ESCs, a normal karyotype, and were positive for ESCs markers including NANOG, REX1, OCT4, TRA-1–60, TRA-1–80, SSEA-3, and SSEA-4. In addition, iPSCs from MSCs of umbilical cord have been generated in a feeder layer-free process using a mini-circle vector containing the reprogramming genes Lin28, Nanog, Oct4 and Sox2 [59]. More recently iPSCs from MSCs of Wharton’s jelly have been generated using genome non-integrating Sendai virus [60].
Conclusion
The possibility of reprogramming adult somatic cells from patients affected with genetic disorders into a virtually inexhaustible reserve of pluripotent stem cells promises a deeper understanding of the malfunctioning of our genes and the cells types affected by the gene mutations. However, the various protocols employed for reprogramming adult somatic cells suffer from several technical roadblocks vis-à-vis low efficiency, extended duration and being labor intensive. Alternatively, cells derived from fetal sources (amniotic fluid, placenta and umbilical cord) present to us an equally viable source of reprogramming. As stated above, the fetal derived cells hold several advantages over the adult sources especially in terms of being less prone to aging dependent genetic and epigenetic modifications. It has also been demonstrated that due to their closer proximity to the pluripotent stem cells state, they are amenable to reprogramming within a shorter duration. The advent of targeted genome-editing tools like the CRISPR/Cas9 system is expected to increase the development of in vitro models for investigations into the etiology of rare genetic disorders, toxicological screening of drug candidates, as well as for gene corrected autologous cell replacement therapy. It can be envisaged that reprogrammed fetal derived cells would play a significant role in this context of pre-clinical screening and disease modeling.
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, et al. Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem Cell. 2009; 5: 111–123.
- Phanstiel DH, Brumbaugh J, Wenger CD, Tian S, Bolin JM, Ruotti V, et al. Proteomic and phosphoproteomic comparison of human ES and iPS cells. Nat Methods. 2012; 8: 821–827.
- Munoz J, Low TY, Kok YJ, Chin A, Frese CK, Ding V, et al. The quantitative proteomes of human-induced pluripotent stem cells and embryonic stem cells. Mol Syst Biol. Nature Publishing Group. 2011; 7: 1–13.
- Faradonbeh MZ, Gharechahi J, Mollamohammadi S, Pakzad M, Taei A, Rassouli H, et al. An orthogonal comparison of the proteome of human embryonic stem cells with that of human induced pluripotent stem cells of different genetic background. Mol Biosyst. 2012; 8: 1833-1840.
- Narsinh KH, Plews J, Wu JC. Comparison of human induced pluripotent and embryonic stem cells: fraternal or identical twins? Mol Ther. Nature Publishing Group. 2011; 19: 635–638.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126: 663–676.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131: 861–872.
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318: 1917–1920.
- Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS One. 2011; 6: e17557.
- Lu J, Kong X, Luo C, Li KK. Application of Epigenome-Modifying Small Molecules in Induced Pluripotent Stem Cells. Med Res Rev. 2013; 33: 790–822.
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. Nature Publishing Group. 2009; 6: 805–808.
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-Specific Induced Pluripotent Stem Cells. Cell. 2008; 134: 877–886.
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007; 318: 1920–1923.
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008; 105: 5856–5861.
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011; 8: 409–412.
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008; 26: 1276–1284.
- Jeong BK, Holm Z, Araúzo-Bravo MJ, Hans RS. Generation of induced pluripotent stem cells from neural stem cells. Nat Protoc. 2009; 4: 1464–1470.
- Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang F, et al. Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differentiation. 2010; 80: 123–129.
- Seki T, Yuasa S, Fukuda K. Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nat Protoc. Nature Publishing Group. 2012; 7: 718–728.
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, et al. Generation of Pluripotent Stem Cells from Adult Mouse Liver and Stomach Cells. Science. 2008; 321: 641.
- Mukherjee S, Thrasher AJ. IPSCs: Unstable origins? Mol Ther. Nature Publishing Group. 2011; 19: 1188–1190.
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011; 29: 1117–1119.
- Li G, Zhang XA, Wang H, Wang X, Meng CL, Chan CY, et al. Comparative proteomic analysis of mesenchymal stem cells derived from human bone marrow, umbilical cord, and placenta: implication in the migration. Proteomics. 2009; 9: 20–30.
- Shaw SWS, David AL, De Coppi P. Clinical applications of prenatal and postnatal therapy using stem cells retrieved from amniotic fluid. Curr Opin Obstet Gynecol. 2011; 23: 109–116.
- Ye L, Chang JC, Lin C, Sun X, Yu J, Kan YW. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. PNAS. 2009; 106: 9826–9830.
- Pipino C, Di Tomo P, Mandatori D, Cianci E, Lanuti P, Cutrona MB, et al. Calcium sensing receptor activation by calcimimetic R-568 in human amniotic fluid mesenchymal stem cells: correlation with osteogenic differentiation. Stem Cells Dev. 2014; 23: 2959–2971.
- Pipino C, Pierdomenico L, Di Tomo P, Di Giuseppe F, Cianci E, D’Alimonte I, et al. Molecular and Phenotypic Characterization of Human Amniotic Fluid-Derived Cells: A Morphological and Proteomic Approach. Stem Cells Dev. 2015; 24: 1415–1428.
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007; 25: 100–106.
- Pipino C, Mukherjee S, David AL, Blundell MP, Shaw SW, Sung P, et al. Trisomy 21 Mid-Trimester Amniotic Fluid Induced Pluripotent Stem Cells Maintain Genetic Signatures During Reprogramming: Implications for Disease Modeling and Cryobanking. Cell Reprogram. 2014; 16: 331–344.
- Anchan RM, Quaas P, Gerami-Naini B, Bartake H, Griffin A, Zhou Y, et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet. 2011; 20: 962–974.
- Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet. 2009; 18: 4340–4349.
- Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G, et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram. 2010; 12: 117–125.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872.
- Wolfrum K, Wang Y, Prigione A, Sperling K, Lehrach H, Adjaye J. The LARGE principle of cellular reprogramming: lost, acquired and retained gene expression in foreskin and amniotic fluid-derived human iPS cells. PLoS One. 2010; 5: e13703.
- Li Q, Fan Y, Sun X, Yu Y. Generation of induced pluripotent stem cells from human amniotic fluid cells by reprogramming with two factors in feeder-free conditions. J Reprod Dev. 2013; 59: 72–77.
- Fan Y, Luo Y, Chen X, Li Q, Sun X. Generation of Human β-thalassemia Induced Pluripotent Stem Cells from Amniotic Fluid Cells Using a Single Excisable Lentiviral Stem Cell Cassette. J Reprod Dev. 2012; 58: 404-409.
- Mukherjee S, Pipino C, David AL, De Coppi P, Thrasher AJ. Emerging neuronal precursors from amniotic fluid-derived down syndrome induced pluripotent stem cells. Hum Gene Ther. 2014; 25: 682–683.
- Spitalieri P, Talarico RV, Botta A, Murdocca M, D’Apice MR, Orlandi A, et al. Generation of Human Induced Pluripotent Stem Cells from Extraembryonic Tissues of Fetuses Affected by Monogenic Diseases. Cell Reprogram. 2015; 17: 275–287.
- Liu T, Zou G, Gao Y, Zhao X, Wang H, Huang Q, et al. High Efficiency of Reprogramming CD34(+) Cells Derived from Human Amniotic Fluid into Induced Pluripotent Stem Cells with Oct4. Stem Cells Dev. 2012; 21: 2322–2332.
- Qin M, Chen R, Li H, Liang H, Xue Q, Li F, et al. Direct reprogramming of human amniotic fluid stem cells by OCT4 and application in repairing of cerebral ischemia damage. Int J Biol Sci. 2016; 12: 558–568.
- Slamecka J, Salimova L, McClellan S, Kelle van M, Kehl D, Laurini J, et al. Non-integrating episomal plasmid-based reprogramming of human amniotic fluid stem cells into induced pluripotent stem cells in chemically defined conditions. Cell Cycle. 2016; 15: 234–249.
- Moschidou D, Mukherjee S, Blundell MP, Drews K, Jones GN, Abdulrazzak H, et al. Valproic Acid Confers Functional Pluripotency to Human Amniotic Fluid Stem Cells in a Transgene-free Approach. Mol Ther. 2012; 20: 1953–1967.
- Moschidou D, Mukherjee S, Blundell MP, Jones GN, Atala AJ, Thrasher AJ, et al. Human Mid-Trimester Amniotic Fluid Stem Cells Cultured Under Embryonic Stem Cell Conditions with Valproic Acid Acquire Pluripotent Characteristics. Stem Cells Dev. 2012; 22: 444-458.
- Pipino C, Shangaris P, Resca E, Zia S, Deprest J, Sebire NJ, et al. Placenta as a reservoir of stem cells: an underutilized resource? Br Med Bull. 2013; 105: 43–68.
- 45. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006; 2: 133–142.
- Delo DM, De Coppi P, Bartsch G, Atala A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006; 419: 426–438.
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, et al. Engraftment Potential of Human Amnion and Chorion Cells Derived from Term Placenta. Transplantation. 2004; 78: 1439–1448.
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005; 23: 1549–1559.
- Lichtner B, Matz P, Adjaye J. Generation of iPSC lines from primary human chorionic villi cells. Stem Cell Res. 2015; 15: 697–699.
- Easley CA, Miki T, Castro CA, Ozolek JA, Minervini CF, Ben-Yehudah A, et al. Human Amniotic Epithelial Cells are Reprogrammed More Efficiently by Induced Pluripotency than Adult Fibroblasts. Cell Reprogram. 2012; 14: 193–203.
- Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, et al. Functional genomics reveals a BMP-Driven mesenchymal-to-Epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010; 7: 64–77.
- Abdulrazzak H, Moschidou D, Jones G, Guillot P V. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010; 7: S689–S706.
- Broxmeyer HE. Cord blood hematopoietic stem cell transplantation. StemBook. 2010;1–14.
- Giorgetti A, Montserrat N, Aasen T, Haase A, Olmer R, Schwanke K, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009; 5: 353–357.
- Bieback K, Kern S, Klüter H, Eichler H. Critical Parameters for the Isolation of Mesenchymal Stem Cells from Umbilical Cord Blood. Stem Cells. 2004; 22: 625–634.
- Secco M, Zucconi E, Vieira NM, Fogaça LLQ, Cerqueira A, Carvalho MDF, et al. Mesenchymal stem cells from umbilical cord: do not discard the cord! Neuromuscul Disord. 2008; 18: 17–18.
- Fu Y-S, Cheng Y-C, Lin M-YA, Cheng H, Chu P-M, Chou S-C, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006; 24: 115–124.
- Cai J, Li W, Su H, Qin D, Yang J, Zhu F, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem. 2010 ; 285: 11227–11234.
- . Daneshvar N, Rasedee A, Shamsabadi FT, Moeini H, Mehrboud P, Rahman HS et al. Induction of pluripotency in human umbilical cord mesenchymal stem cells in feeder layer-free condition. Tissue Cell. 2015; 47: 575–82.
- Miere C, Devito L, Ilic D. Sendai Virus-Based Reprogramming of Mesenchymal Stromal/Stem Cells from Umbilical Cord Wharton’s Jelly into Induced Pluripotent Stem Cells. Methods Mol Biol. 2016; 1357: 33–44.

Sign up for Article Alerts