Clearing Up the Confusion: An Unsuspected Case of Primary Sclerosing Encapsulating Peritonitis (Cocoon Abdomen) Treated By Laparoscopy
Weber Sánchez Alejandro*, Garteiz Martínez Denzil, Jafif Marcos and Flores-Huidobro AM
Department of Surgery, Hospital Angeles Lomas, Huixquilucan, Estado de México
*Address for Correspondence: Weber Sanchez Alejandro, Departamento de Cirugia Hospital Angeles Lomas. Vialidad de la Barranca 240, Hacienda de las Palmas, 52763 Huixquilucan, México, Tel: +52-555-246-9527; E-mail: awebersanchez@gmail.com
Submitted: 11 October 2019; Approved: 21 October 2019; Published: 23 October 2019
Citation this article: Weber SA, Garteiz MD, Jafif CM, Flores-Huidobro AM. Clearing Up the Confusion: An Unsuspected Case of Primary Sclerosing Encapsulating Peritonitis (Cocoon Abdomen) Treated By Laparoscopy. Open J Surg. 2019;3(2): 034-039.
Copyright: © 2019 Weber SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Sclerosing encapsulating peritonitis; Abdominal cocoon; Cocoon syndrome; Laparoscopy; Laparoscopic surgery; Intestinal obstruction; Abdominal pain
Download Fulltext PDF
Sclerosing Encapsulating Peritonitis (SEP) or cocoon abdomen is a rare inflammatory condition, usually of unknown origin, known by different names which give rise to confusion. In severe cases, an intestinal occlusion can occur due to the membrane that encapsulates the bowel. Being a rare and clinical nonspecific condition, it is commonly under-diagnosed thus, it is important for surgeons to be aware of its existence, since it can result in complications that may affect quality of life or even cause death. We present an unsuspected case of cocoon abdomen in a patient scheduled for another surgical procedure and its surgical management by laparoscopy, as well as discussion about the approach, review of the literature and a proposal for a simplified terminology for better understanding.
Introduction
Sclerosing encapsulated peritonitis is a rare peritoneal inflammatory condition usually of unknown etiology, although it has been associated with other medical conditions causing peritoneal irritation and fibroplastic proliferation. It is also identified with other names as Peritoneal Encapsulation (PE), peritonitis chronica fibrosa incapsulata, fibroplastic peritonitis or more commonly with the descriptive name of abdominal cocoon abdomen, because the bowel loops become encapsulated within a thick fibrous peritoneal membrane in the abdominal cavity that sometimes may lead to an intestinal occlusion [1].
It typically affects the small bowel, although it can include other abdominal structures. Being a rare clinical condition, it is commonly underdiagnosed in the early phases. It can produce recurrent episodes of bowel distention or even intestinal obstruction, requiring surgery that usually establishes the accurate diagnosis, and solves the problem by removing the adhesions and releasing the bowel from the constricting fibrous membrane [2,3].
The objective of this report is to present a case of unsuspected cocoon syndrome incidentally discovered in a patient scheduled for a laparoscopic antireflux surgery and solved during the procedure, a review of the literature and a proposal for a simplified terminology for better understanding.
Case Report
A 22 year-old female, with history of Gastroesophageal Reflux Disease (GERD) and persistent severe abdominal wall pain with a one centimeter hard fixed mass, located at the left semilunar line, 3 cm below the level of the umbilicus, suggestive of Spiegel’s hernia by ultrasound. Additionally, the patient referred long-standing recurrent episodes of colicky abdominal pain, distension and occasional sub-occlusive symptoms needing hospital admission without surgery usually after heavy meals, diagnosed years before as Irritable Bowel Syndrome (IBS). She had also history of left laparoscopic inguinal hernia repair and an umbilicoplasty with synthetic mesh placement performed without complications one year before. Endoscopic findings showed evidence of a hiatal hernia and grade C reflux esophagitis, so the patient was offered laparoscopic surgical treatment to solve the supposed Spiegel and hiatal hernia and GERD. At the time of surgery, patient was asymptomatic and physical exam was negative except for the localized pain in the left semilunar line.
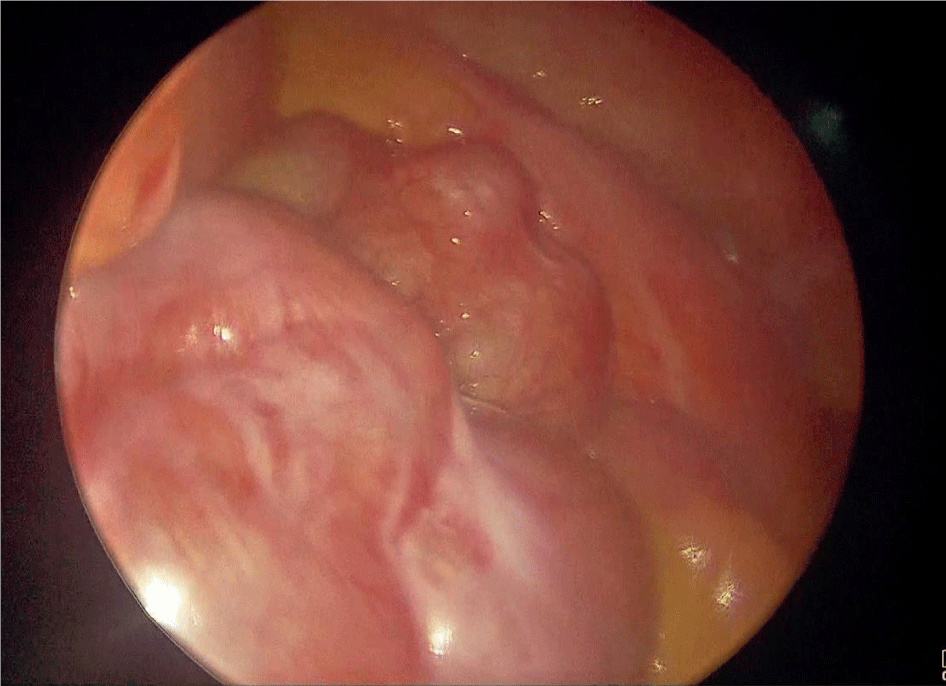
Pneumoperitoneum was achieved uneventfully through a closed technique with Veress needle and a 10 mm trocar was inserted. Initial laparoscopy evidenced multiple firm adhesions between intestinal loops and the abdominal wall, as well as clusters of distended small bowel loops encapsulated within a whitish thick fibrous membrane indicative of SEP. Four other 5 mm trocars were inserted for the procedure (Figure 1).
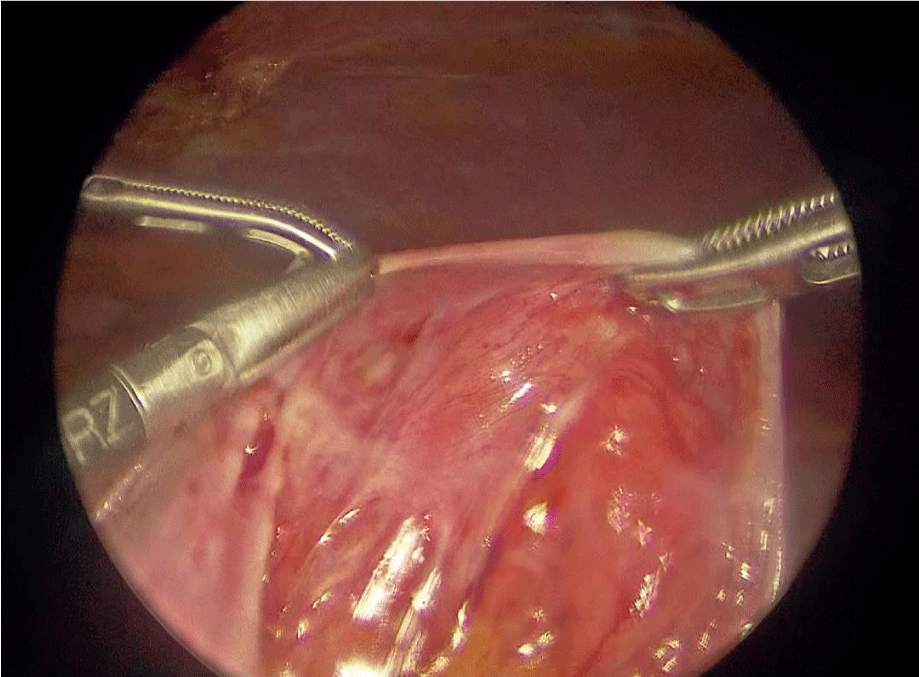
An extensive adhesiolysis was performed to release all the intestinal loops enclosed in the membranes, which affected multiple portions of the intestine. The thick membranes were resected from the bowel whenever possible, since they were firmly attached, and tissue obtained from the membranes was sent to pathology (Figure 2 & 3).
At the site where the patient referred the abdominal wall pain, a granuloma enclosed within the abdominal wall, formed by one of the corner suture fixations of the mesh which covered the umbilical repair was seen and removed along with the suture. Finally, the surgical procedure continued as planned, performing the hiatal hernia repair and Nissen fundoplication as scheduled.
Histopathological report of the resected membranous tissue was peritoneal dense fibrosclerosis, vascular congestion with evident peri-vasculitis and edema, areas of dense sclerosis of the peritoneal stroma with inflammatory cells predominantly neutrophilic polymorphonuclear, and the absence of mesothelial cells suggestive of congenital dysplasia confirmed the diagnosis of Cocoon syndrome (Figure 4).
The patient was initiated on liquids the day after and discharged uneventfully 48hs after surgery. On follow-up, the patient referred complete relief of reflux symptoms, as well as the abdominal wall pain at semilunar line, and eight months after the procedure she remains symptom free without sub-occlusive symptoms, nausea or vomit.
Discussion
Sclerosing encapsulating peritonitis, first defined in 1907 by Owtschinnikow to describe encasement of the intestines by a fibrocollagenous membrane, or cocoon abdomen as named by Foo in 1978, was recognized more than a century ago by the name of peritonitis chronica fibrosa incapsulata [4-7]. It is also known with the names of fibroblastic peritonitis, or idiopathic encapsulating peritoneal sclerosis to identify this rare disease characterized by partial or total encasement of the bowel by an abnormal thick, fibrous membrane lining the intestine causing abdominal discomfort and occlusive or sub-occlusive symptoms. These various given names are used interchangeably in many previously published articles, creating confusion. Recently, Akbulut [8] proposed to simplify this situation distinguishing primary SEP (idiopathic, abdominal cocoon syndrome: patients with no factors explaining SEP) or secondary forms of SEP, but until now this proposition has not been widely accepted, so misunderstanding unfortunately continues.
Most of the cases reported are isolated reports as the one presented here, and to date, very few small series have been described. Akbulut et al, revised the literature searching all the related terms from January 2000 to May 2014, selecting 73 articles [8]. India, Turkey, Nigeria and China have the greatest number of articles. The largest series reported mainly Chinese, suggest that this problem may develop in men, women, and less likely in children, with mean age of presentation around the third decade of life. It seems also that there is no clear gender prevalence, but idiopathic SEP is twice as common in men as in women [2,9].
The idiopathic condition has frequently been described in puberty females from tropical and sub-tropical regions as India, China, Malaysia, Singapore, Nigeria, Kenya and South Africa [10]. Secondary SEP is more common than the idiopathic form; chronic inflammation due to Peritoneal Dialysis (PD) is the most known frequent trigger, with a rate of 1.5% of patients on long-term PD as reported by United Kingdom [11]. Other factors as fungal or viral abdominal infection, tuberculosis, cirrhosis, retrograde menstruation peritonitis especially when associated to infection, intake or exposure to some medications as β-blockers or methotrexate, asbestosis, autoimmune causes or surgery have also been related [12-18]. A case of meconium peritonitis in a neonate has also been reported, although SEP or abdominal cocoon is extremely rare in neonates and infants [19]. In the case we present here there were no apparent causal factor; previous surgery she had was apparently uneventful and unlikely to be the cause of cocoon, but it is noteworthy that surgeons who did the first procedure elsewhere didn’t report the encapsulation of the bowel to the patient or her parents.
It is important to note that there is a congenital related condition, different from SEP, known long time ago as Peritoneal Encapsulation (PE) described by Cleland in 1868 [20] in which bowel is also encased by a membrane, but the peritoneal covering originates from the yolk sac during the return process of organs to the abdomen during embryological development as was proposed by Papez, not related to an inflammatory process like SEP, but to an anatomic developmental anomaly [21,22]. The mesothelial membrane is usually found between the mesocolon and omentum, and most of the small bowel lie posterior to it. PE is typically asymptomatic and usually detected incidentally during laparotomy performed for other indications [20,23,24].
The names used in literature to all these related conditions are often given indistinctly leading to confusion and misunderstanding [25]. It would probably be better, and we suggest therefore to name all these related conditions only with the one familiar and descriptive name of Cocoon abdomen, indicating and focusing on the encapsulating condition, although we can distinguish and is worth to do, between the primary, secondary or congenital nature from the standpoint of view of the etiology.
Exact pathogenetic mechanism of SEP remains unknown although some hypotheses have been postulated. It has been proposed that in cases of primary SEP, the fibrous membrane that encases the intestines is the result of a developmental disorder, suggesting vascular anomalies and omental hypoplasia [26].
Experimental coeliac irrigation with acidic solution or powder has been used in animal models to reproduce dialysis-associated SEP [27,28]. On the other hand, secondary SEP seems to be the result of chronic peritoneal inflammation produced by the known triggers, which causes fibrotic proliferation, hyperplasia of peritoneal mesothelial cells and an abnormal process of peritoneal capillary angiogenesis [29]. Omentum dysplasia was found in 41.7% of the series of SEP reported by Wei, [9] and it has been hypothesized that in other cases, chronic peritonitis results in profuse exudation and malabsorption of fibrin in the abdominal cavity, with fibrin deposits and hyperplasia of the connective tissue forming the membranes and encapsulation.
In secondary SEP due to PD, by far one of the most frequent forms, pathogenesis is probably related to glucose exposure or glucose degradation products in the dialysis fluid [30,31]. It has been suggested that cases associated with organ transplantation may be related to the cessation of dialysis itself, since once the inflammation process has begun, PD lavage can limit the accumulation of fibrin [32,33]. In addition, calcineurin inhibitors, which are an anti-rejection medication commonly used in transplant surgery are profibrotic, exacerbating the fibrin deposition process [34,35].
The SEP membrane is characteristically a thick, whitish, opaque fibrous layer which contains inflammatory cells, unlike the membrane of PE which is formed by mesothelium. Histopathologically, the peritoneum of patients with SEP is characterized by fibroconnective tissue proliferation, inflammatory infiltration, and dilated lymphatics. No foreign body granulomas, giant cells, or birefringent material is present. Garosi and colleagues reported thickening of the submesothelial cell layer, vasculopathy, inflammation, arterial occlusion, and tissue and arterial calcification [36,37].
SEP may enclose the intestine by segments or completely, and may occasionally involve other intraperitoneal organs including the stomach, liver or colon. To better characterize the disease extension, Cocoon syndrome has been classified by some into three categories based on the magnitude of the membrane lining. Type I and II involve encapsulation of part or the entire small bowel length respectively. In type III, the appendix, the cecum, ascending colon, stomach, liver and the ovaries may also be affected in addition to the small intestine [8,32]. According to this classification the patient we report was type II because the entire small bowel by segments was affected but colon, stomach, liver and spleen were not encased.
Signs and symptoms are related to the degree of intestinal imprisonment by the membrane. It seems to be a slowly progressive condition initially asymptomatic, that in secondary cases may develop several years after the exposure to the triggering factor when it is known. Patient’s medical history (peritoneal dialysis, tuberculosis, systemic lupus erythematosus, abdominal and pelvic pain that occurs repeatedly midway through a woman’s menstrual cycle.) provides important clues to consider secondary SEP. Patients with recurrent attacks of abdominal pain not explained by other means may be carriers of primary SEP [6].
Different clinical stages of SEP may be identified as the disease progresses. Patients in early stages are asymptomatic or the signs and symptoms are mild and nonspecific and may include anorexia, nausea, abdominal discomfort, diarrhea and intermittent abdominal pain. Physical examination is also usually nonspecific.
Patients diagnosed as SEP, with minimal symptoms may have conservative management through decompression with a nasogastric tube, intestinal rest and enteral or parenteral nutrition if unable to eat for long periods. Medical treatment with tamoxifen, steroids, azathioprine and mycophenolate mofetil and colchicine may be effective in patients with idiopathic SEP resistant to conservative therapy [38,39] however, most patients eventually require surgery to perform extensive adhesiolysis and resection of the membrane that covers the intestines, in order to avoid recurrence of intestinal occlusion [1,8,40].
Patients with the late stage of the diseases, usually show up to the emergency room when they present abdominal pain, signs and symptoms of acute, subacute or chronic attacks of gastrointestinal obstruction, malnutrition and malaise [41]. On physical examination two signs of SEP were described by Naraynsingh et al, a fixed asymmetrical distension of the abdomen which does not vary with peristaltic activity due to the fibrous capsule, and difference in the consistency of the abdominal wall to palpation due to the intestinal encasement [42]. A firm or soft palpable mass is often reported, as 51% of 82 SEP cases in the series reported by Xia et al. [2] reviewing the Chinese Biology and Medicine Database in ten years. Of the total of 82 cases, 54.9% presented intestinal obstruction, 17.1% acute appendicitis and intestinal perforation or necrosis in 11%. Female patients may be affected with infertility because layer fibrous tissue wrap the bowel and also the reproductive organs, in this series 18.3% were reported to have infertility [2,11,43]. Some cases may manifest with uncommon, but life-threatening complications including enteroatmospheric fistula or small bowel necrosis [8].
There are no laboratory findings characteristic of SEP. Peritoneal dialysis fluid may show leukocytosis. Peritoneal equilibrium tests may show a trend towards greater solute transport and reduced ultrafiltration, specifically with lower rates of free water transport [44-46]. However, these changes are not characteristic and are commonly observed in the absence of SEP, particularly among patients who have been on PD for several years.
Image studies are usually inconclusive. Plain X-rays may show diffuse dilated small intestinal loops and air-fluid levels non-specific of SEP, but sometimes the wall of the “cocoon” may be seen calcified. Barium studies are uncommon because they are not indicated in patients with intestinal obstruction, but in subacute cases they may show clusters of intestinal loops together as within a sac, giving a cauliflower-like appearance on sequential films [47]. Ultrasonography may show intestinal segments clustered and encased by a hyperechoic dense membrane [48]. Computed Tomography (CT) especially multidetector technology gives more accurate information and can identify better dilatation of intestinal loops, intestinal wall thickening, type of bowel involvement, and sometimes the fibrous membrane being intestinal volvulus and peritoneal membrane calcification the most specific findings. MR enterography although seldom reported for the diagnosis of SEP is probably similar to or even better than CT-acquired images [30,31,49,50].
Differential diagnosis should be made with other causes of intestinal obstruction such as internal herniation and congenital PE that should be primarily considered in these patients [51]. Other conditions are voluminous invagination, intestinal malrotation, secondary peritonitis and tuberculous peritonitis, mainly in patients who live in tuberculosis-prevalent regions [8,52].
Most of physicians and surgeons have never seen patients with SEP, and being a rare condition preoperative diagnosis is extremely difficult because it requires a high index of clinical suspicion [53]. Less than fifty percent of the patients are recognized as SEP preoperatively [54,55].
Usually the only way to confirm the diagnosis is by laparoscopy or laparotomy giving the opportunity to solve the problem definitively at the same time [56]. Ideal treatment of SEP is surgery, releasing the adhesions and excising the constricting fibrous membrane from the small intestine.
Resection of the bowel is only indicated if a segment is nonviable, because it increases morbidity and mortality. The long-term prognosis after surgery is typically excellent, but complications may occur as 23.2% of the operated cases reviewed by Xia et al. presented postoperative bowel obstruction which is the most common complication, six cases in this series developed an intestinal fistula and 3 of them died [2,13].
Sometimes SEP is detected incidentally during a surgical procedure performed for other purpose as the case we present here because she was diagnosed with IBS and no intestinal abnormalities were reported in the previous surgery the abdominal symptoms were attributed to this condition, but when the intestinal adhesions and fibrous membrane encasing bowel loops were seen, intraoperative diagnosis was made and adhesiolysis and resection of intestinal membranes were laparoscopically done. Although laparotomy has been the procedure of choice in the majority of the reported SEP cases, lately few cases of patients with Cocoon abdomen treated by laparoscopy have been reported. We think that whenever possible, laparoscopy done by experienced surgeons seems to be the method of choice to perform adhesiolysis and intestinal membrane resection because of the advantages of mini-invasive surgery and good results, avoiding further adhesions more predictable in open surgery. [57-60] Special care during laparoscopic procedure is needed. Open induction of pneumoperitoneum especially in cases of intestinal occlusion would be a safer option. Tissue dissection should be gentle and careful adhesiolysis and resection of the membrane with scissors is better than with electrosurgery to avoid bowel damage. In the case of this patient in whom the scheduled surgery was hiatal hernia repair, Nissen fundoplication and revision of the pain site supposed to be a Spigelian hernia, laparoscopy gave us the opportunity to solve the cocoon and perform the procedures, hiatal hernia repair, Nissen fundoplication and resection of the abdominal wall granuloma uneventfully as scheduled, with amazing recovery and excellent results.
Conclusion
Despite the low incidence of SEP or cocoon abdomen, it is important for surgeons to be aware of the existence of this pathology in order to identify it when necessary. Laparoscopic treatment in selected cases may be the procedure of choice because of the advantages of miniinvasive surgery. We make a proposal for a simplified terminology to avoid misunderstanding and confusion between the multiple names given to this condition.
- Machado NO. Sclerosing encapsulating peritonitis. Sultan Qaboos Univ Med J. 2016; 6: 142-151. https://bit.ly/32yke9f
- Xia J, WeiJia X, Chen L, Liu D. Abdominal cocoon with early postoperative small bowel obstruction. A case report and review of literature in China. Medicine. 2018; 97: e11102. https://bit.ly/2BFGoux
- Li N, Zhu W, Li Y, Gong J, Gu L, Li M, et al. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014; 38: 1860-1867. https://bit.ly/33OCprw
- Choudhury T, Kamal M. Abdominal Cocoon - A Case Report with Short Review of Literature. BSMMU J. 2009; 2: 81-84. https://bit.ly/35ROgqr
- Tasdelen N, Demirag A, Kalayci M, Gurses M, Kilickesmez NO, Comunoglu N, et al. Intestinal obstruction due to abdominal cocoon: CT findings. Eur J Radiol. 2009; 70: 79-81. https://bit.ly/2MzTNKS
- Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012; 18: 1999-2004. https://bit.ly/33MHbWo
- Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978; 65: 427-430. https://bit.ly/2pB14AU
- Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015; 21: 675-687. https://bit.ly/2J6DMKf
- Wei B, Wei HB, Guo WP, Zheng ZH, Huang Y, Hu BG, et al. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009; 198: 348-353. https://bit.ly/2qrcHuA
- Hossain M, Ahamed B, Nargis W. A study of the epidemiology and outcome of patients with acute intestinal obstruction due to abdominal cocoon; an experience of a tertiary hospital from a tuberculosis endemic third world country. J Basic Med Allied Health Sci. 2012; 2: 5.
- Brown MC, Simpson K, Kerssens JJ, Mactier RA. Encapsulating peritoneal sclerosis in the new millennium: A National cohort study. Clin J Am Soc Nephr. 2009; 4: 1222-1229. https://bit.ly/2J6Ebfq
- Devay AO, Gomceli I, Korukluoglu B, Kusdemir A. An unusual and difficult diagnosis of intestinal obstruction: The abdominal cocoon. Case report and review of the literature. World J Emerg Surg. 2006; 24: 1-8. https://bit.ly/2J8DZg1
- Grefberg N, Nilsson P, Andreen T. Sclerosing obstructive peritonitis, beta-blockers and continuous ambulatory peritoneal dialysis. Lancet. 1983; 322: 733-734. https://bit.ly/2pAFKLU
- Yamamoto S, Sato Y, Takeishi T, Kobayashi T, Hatakeyama K. Sclerosing encapsulating peritonitis in two patients with liver cirrhosis. J Gastroenterol. 2004; 39: 172-175. https://bit.ly/2P0JDo9
- Lalloo S, Krishna D, Maharajh J. Case report: abdominal cocoon associated with tuberculous pelvic inflammatory disease. Br J Radiol. 2002; 75: 174-176. https://bit.ly/2PfoPJN
- Fowler R. Primary peritonitis-changing aspects, 1956-1970. Aust Pediatr J. 1971; 7: 73-83. https://bit.ly/32xBxax
- Oran E, Seyit H, Besleyici C, Ünsal A, Alış H. Encapsulating peritoneal sclerosis as a late complication of peritoneal dialysis. Ann Med Surg. 2015; 4: 205-207. https://bit.ly/2oPZD1E
- Maguire D, Srinivasan P, O’Grady J, Rela M, Heaton ND. Sclerosing encapsulating peritonitis after orthotropic liver transplantation. Am J Surg. 2001; 182:151-154. https://bit.ly/32yUrxI
- Ahmad S, Kayastha K, Javed S, Wasti A. Abdominal cocoon secondary to meconium peritonitis in a neonate: a case report. J Neonat Surg. 2013; 2: 12. https://bit.ly/2pH19CY
- Browne LP, Patel J, Guillerman RP, Hanson IC, Cass DL. Abdominal cocoon: a unique presentation in an immunodeficient infant. Pediatr Radiol. 2012; 42: 263-266. https://bit.ly/2P5grwj
- Papez JW. A rare intestinal anomaly of embryonic origin. Anat Ret. 1932; 54: 197-214. https://bit.ly/2pzuFuG
- Batson OV. Anatomic variation in the abdomen. Surg Clin North Am. 1955; 35: 1527-1537. https://bit.ly/32z2tXp
- Kaur R, Chauhan D, Dalal U, Khurana U. Abdominal cocoon with small bowel obstruction: two case reports. Abdom Imag. 2011; 37: 275-278. https://bit.ly/35SN7z5
- Basu A, Sukumar R, Sistla SC, Jagdish S. “Idiopathic” abdominal cocoon. Surgery. 2007; 141: 277-278.
- Sahoo AN, Gangopadhyay DK, Gopal SP, Sharma SP, Dash RN. Abdominal cocoon in children: a report of four cases. J Pediatr Surg. 1996; 31:987-988. https://bit.ly/2BFKBOR
- Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol. 2007; 13: 3649-3651. https://bit.ly/2MX2JbX
- Levine S, Saltzman A. Abdominal cocoon: an animal model for a complication of peritoneal dialysis. Perit Dial Int. 1996; 16: 613-616. https://bit.ly/35QqKKz
- Cudazzo E, Lucchini A, Puviani PP, Dondi D, Binacchi S, Bianchi M, et al. Sclerosing peritonitis: a complication of LeVeen peritonevenous shunt. Minerva Chir. 1999; 54: 809-812. https://bit.ly/2VXDhHm
- Dobbie J. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit Dial Int. 1992; 12: 14-27. https://bit.ly/33I3Ov0
- Brown EA, Bargman J, Van Biesen W, Chang M-Y, Finkelstein FO, Hurst H, et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis-position paper for ISPD: 2017 Update. Perit Dial Int. 2017; 37: 362-374. https://bit.ly/2MZttsh
- Kawanishi H, Shintaku S, Banshodani M, Hashimoto S. Nitta K. Past and present perspectives on encapsulating peritoneal sclerosis. Chronic kidney diseases-recent advances in clinical and basic research. Basel, Karger. 2015; 185: 87-97. https://bit.ly/2MWgt72
- Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int. 2010; 77: 904-912. https://bit.ly/33MVE4z
- Korte MR, Yo M, Betjes MG, Fieren MW, van Saase JC, Boer WH, et al. Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol Dial Transplant. 2007; 22: 2412-2414. https://bit.ly/2qwurF1
- Fieren MW, Betjes MG, Korte MR, Boer WH. Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int. 2007; 27: 619-624. https://bit.ly/35SPWjq
- de Freitas D, Augustine T, Brenchley P, Brown E, Collinson H, Davenport A, et al. Encapsulating Peritoneal Sclerosis Following Renal Transplantation-The UK Experience: Abstract #67. Am J Transplant. 2007; 7: 164.
- Garosi G, Di Paolo N, Sacchi G, Gaggiotti. Sclerosing peritonitis: a nosological entity. Perit Dial Int. 2005; 25: 110-112. https://bit.ly/32xX2Yt
- Tombak MC, Apaydin FD, Colak T, Duce MN, Balci Y, Yazici M, et al. An unusual cause of intestinal obstruction: abdominal cocoon. Am J Roent. 2010; 194: 176-178. http://bit.ly/2pzpbQD
- Solak A, Solak İ. Abdominal cocoon syndrome: preoperative diagnostic criteria, good clinical outcome with medical treatment and review of the literature. Turk J Gastroenterol. 2012; 23: 776-779. http://bit.ly/31yiCuO
- Cornelis T, Oreopoulos DG. Update on potential medical treatments for encapsulating peritoneal sclerosis; human and experimental data. Int Urol Nephrol. 2011; 43: 147-156. http://bit.ly/32youp5
- Li N, Zhu W, Li Y, Gong J, Gu L, Li M, et al. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014; 38: 1860-1867. http://bit.ly/2VYTrAp
- Nakamoto H. Encapsulating peritoneal sclerosis a clinician's approach to diagnosis and medical treatment. Perit Dial Int. 2005; 25: 30-38. http://bit.ly/35VgMrs
- Naraynsingh V, Maharaj D, Singh M, Ramdass MJ. Peritoneal encapsulation: a preoperative diagnosis is possible. Postgrad Med J. 2001; 77: 725-726. http://bit.ly/2Bupw9L
- Hu YJ, Zhu YM. Laparoscopic examination and treatment of abdominal cocoon with infertility: a report of 6 cases. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2004; 33: 462-464. http://bit.ly/2MWstoZ
- Morelle J, Sow A, Hautem N, Bouzin C, Crott R, Devuyst O, et al. Interstitial fibrosis restricts osmotic water transport in encapsulating peritoneal sclerosis. J Am Soc Nephrol. 2015; 26: 2521-2533. http://bit.ly/2BvEMTM
- Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, et al. Evaluation and management of ultrafiltration problems in peritoneal dialysis. International society for peritoneal dialysis Ad Hoc committee on ultrafiltration management in peritoneal dialysis. Perit Dial Int. 2000; 20: S5-21. http://bit.ly/2qu8qqh
- Rippe B, Venturoli D. Simulations of osmotic ultrafiltration failure in CAPD using a serial three-pore membrane/fiber matrix model. Am J Physiol Renal Physiol. 2007; 292: F1035-1043. http://bit.ly/35STqm0
- Navani S, Shah P, Pandya S, Doctor N. Abdominal cocoon the cauliflower sign on barium small bowel series. Indian J Gastroenterol. 1995; 14: 19. http://bit.ly/31uo6Xy
- Duman E, Aslan A, Gunduz N, Inan I. Sclerosing encapsulated peritonitis: typical imaging findings for easy diagnosis. Ann Saudi Med 2018; 38: 230-232. http://bit.ly/2pBnulG
- Gupta S, Shirahatti RG, Anand J. CT Findings of an Abdominal cocoon. AJR Am J Roentgenol. 2004; 183: 1658-1660. http://bit.ly/2o8FJP2
- Tarzi RM, Lim A, Moser S, Ahmad S, George A, Balasubramaniam G, et al. Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol. 2008; 3: 1702-1710. http://bit.ly/32z1dDE
- Serter A, Kocakoç E, Çipe G. Supposed to be rare cause of intestinal obstruction; abdominal cocoon: report of two cases. Clin Imaging. 2013; 37: 586-589. http://bit.ly/2PfwgRd
- Kaushik R, Punia RPS, Mohan H, Attri A. Tuberculous abdominal cocoon – a report of 6 cases and review of the Literature. World J Emerg Surg. 2006; 1: 18. http://bit.ly/32yqei7
- Da Luz MM, Barral SM, Barral CM, Bechara Cde S, Lacerda- Filho A. Idiopathic encapsulating peritonitis: report of two cases. Surg Today 2011; 41: 1644-1648. http://bit.ly/2BuryXr
- Li N, Zhu W, Li Y, Gong J, Gu L, Li M, Cao L, Li J. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg 2014; 38: 1860-1867. http://bit.ly/2VYTrAp
- Singh B, Gupta S. Abdominal cocoon: a case series. Int J Surg 2013; 11: 325-328. http://bit.ly/2J8qRrg
- Kawaguchi Y, Saito A, Kawanishi H, Nakayama M, Miyazaki M, Nakamoto H, et al. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int. 2005; 25: S83-95. http://bit.ly/2VXJe7a
- Makam R, Chamany T, Ramesh S, Potluri VK, Varadaraju PJ, Kasabe P. Laparoscopic management of abdominal cocoon. J Minim Access Surg. 2008; 4: 15-17. http://bit.ly/2Bqx7Gm
- Qasaimeh GR, Amarin Z, Rawshdeh BN, El-Radaideh KM. Laparoscopic diagnosis and management of an abdominal cocoon: a case report and literature review. Surg Laparosc Endosc Percutan Tech. 2010; 20: e169-171. http://bit.ly/2MvaeI9
- Hu YJ, Zhu YM. Laparoscopic examination and treatment of abdominal cocoon with infertility: A report of 6 cases. Zhejiang Da Xue Xue Bao Yi Xue Ban 2004; 33: 462-464. http://bit.ly/2MWstoZ
- Ertem M, Ozben V, Gok H, Aksu E. An unusual case in surgical emergency: abdominal cocoon and its laparoscopic management. J Minim Access Surg 2011; 7: 184-186. http://bit.ly/2P0RUZf

Sign up for Article Alerts