Prognosis of Older Patients with Stage II and III Gastric Cancer
Keishiro Aoyagi*, Naotaka Murakami, Taro Isobe, Taizan Minami, Yuya Tanaka, Hideaki Kaku, Fumihiko Fujita and Yoshito Akagi
Department of Surgery, Kurume University School of Medicine, Japan*Address for Correspondence: Keishiro Aoyagi , Department of Surgery, Kurume University School of Medicine, 67 Asahi-machi, Kurume, Fukuoka 830-0011, Japan, Tel: +819-423-533-11 / +819-423-535-05; Fax: +819-423-407-09; ORCID ID: https://orcid.org/0000-0002-1803-1783; E-mail: keishiro@med.kurume-u.ac.jp
Submitted: 15 January 2020; Approved: 06 February 2020; Published: 08 February 2020
Citation this article: Aoyagi K, Murakami N, Isobe T, Minami T, Tanaka Y, et al. Prognosis of Older Patients with Stage II and III Gastric Cancer. Open J Surg. 2020;4(1): 008-014.
Copyright: © 2020 Aoyagi K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Gastric cancer; Stage II and III; Elderly patients; Adjuvant chemotherapy
Download Fulltext PDF
Introduction: Adjuvant chemotherapy such as S-I is thought to prolong the life expectancy of patients with gastric cancer. The number of older patients with gastric cancer has recently been increasing. Here we examined the prognosis of older patients with stage II or III gastric cancer.
Methods: The study cohort comprises 658 patients with stage II or III gastric cancer who underwent curative surgery from 1994 to 2014 in our institution. From 1994 to 2003 was considered the early phase, whereas from 2004 to 2014 was considered the late phase. The patients were classified by age into under 65 years (Non-Elderly [NE]); 65-74 years (Early Elderly [EE]); and over 74 years (Late Elderly [LE] groups.
Results: Significantly more patients in the late phase than the early phase received S-1. Significantly fewer LE patients than NE or EE patients underwent adjuvant chemotherapy. Significantly more deaths were caused by a comorbidity in patients with stage II disease in the LE group than in the other groups. Overall Survival (OS) of patients with stage II or III disease was significantly lower in the LE group than in the other groups. Disease-Specific Survival (DSS) of patients with stage III disease was significantly higher in the NE group than in the other groups. Both OS and DSS were significantly higher in patients with stage III disease in the late phase than in the early phase. Both OS and DSS of patients with stage III disease were significantly better in the adjuvant than the non-adjuvant group.
Conclusion: Adjuvant chemotherapy such as S-I is thought to prolong the life expectancy of patients with gastric cancer. However, administration of adjuvant chemotherapy to LE patients with stage II disease must be carefully considered because of the high comorbidity-related mortality.
Abbreviations
T1: Tumor Confined to the Sub Mucosa; ACTS-GC: Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer; R0: No Residual Tumor; pStage: Pathological Stage; NE: Non-Elderly; EE: Early Elderly; LE: Late Elderly; OS: Overall Survival; DSS: Disease-Specific Survival
Introduction
Gastric cancer is the fifth most common malignancy and third leading cause of cancer-related death worldwide [1]. Despite advances in diagnosis and treatment, gastric cancer remains the third most frequent cause of cancer-related death in Japan [2,3]. Surgery is the mainstay of treatment for gastric cancer. However, many patients with stage II (including T1) or stage III (moderately advanced) disease develop recurrence, even after curative resection. S-1 (TS-1; Taiho Pharmaceutical, Tokyo, Japan) is an oral fluoropyrimidine preparation combining tegafur, gimeracil, and oteracil potassium [4]. The Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC), a prospective, randomized, phase III trial, demonstrated that surgery plus S-1 treatment is more effective than surgery alone in Japanese patients with stage II/III gastric cancer [2,5]. Therefore, adjuvant chemotherapy such as S-I is commonly administered after curative surgery for stage II and III gastric cancer. Several chemotherapy regimens, including molecular targeted drugs and immune checkpoint inhibitors such as trastuzumab and nivolumab, are included in the Gastric Cancer Treatment Guidelines 2018 for advanced or recurrent gastric cancer on the basis of findings of large clinical trials [6]. These treatments appear to prolong the life expectancy of patients with gastric cancer. However, the number of elderly patients with gastric cancer has recently been increasing. The proportion of people aged ≥ 75 years is growing rapidly, having increased from 1.9% of the world’s population in 1980 to more than 3.3% in 2015 [7]. This phenomenon is especially pronounced in Japan, which has one of the longest life expectancies in the world: 87 years for women and 80 years for men [8]. People ≥ 75 years of age currently comprise 13.5% of the total population of Japan [9]; this age group accounts for 52.6% of men and 41.4% of women with gastric cancer [10]. Many studies of the feasibility and safety of surgical treatment for patients aged ≥ 75 years have been conducted [11-14]. Gastrectomy seems to be tolerable if patients are appropriately selected; however, physical status can differ widely among elderly patients [15]. An individual’s physiological reserve decreases with advancing age, resulting in an increased incidence of postoperative complications [16]. Moreover, postoperative adjuvant chemotherapy is often not administered enough to elderly patients with stage II or III gastric cancer.
The aim of the present study was to compare the frequency of administration of adjuvant chemotherapies and the prognosis of elderly patients with stage II or III gastric cancer with those of younger patients.
Methods
Patients
The study cohort comprised 658 of 766 patients with gastric cancer who had undergone resection at Kurume University Hospital, Kurume, Japan. The eligibility criteria for this study were gastrectomy with R0 resection between January 1994 and December 2014, histologically proven gastric adenocarcinoma, and pStage II or III according to the Japanese Classification, 14th Edition [17]. The exclusion criteria were multiple gastric cancers, remnant gastric cancer, synchronous or metachronous cancer within 1 year after gastric surgery, and a follow-up period of less than 1 month after surgery.
From 1994 to 2003 was considered the early phase, whereas from 2004 to 2014 was considered the late phase. The patients were also classified by age: under 65 years, non-elderly (NE); 65-74 years, Early Elderly (EE); and over 74 years, Late Elderly (LE).
Statistical analysis
The median follow-up time from the date of surgery was 42 (range, 1-165) months for survivors. Three and 5-year survival rates were calculated using the Kaplan-Meier method; the log-rank test was used to assess the significance of intergroup differences.
The following factors were considered: age (NE, EE, or LE), phase (early or late), postoperative chemotherapy (adjuvant + or −), and S-1 administered (S-1 + or −) to patients with stage II and stage III gastric cancer.
Values of p < 0.05 were considered to denote significant differences. The statistical analysis was performed using JMP 13 software (SAS Institute Inc., Cary, NC, USA)
Results
Overall, 423 patients were men and 235 women; the median age was 68 (range, 27-92) years. There were 269 patients with stage II gastric cancer and 389 with stage III cancer. Surgery was performed on 318 patients (stage II, n = 118; stage III, n = 200) in the early phase and 340 (stage II, n = 151; stage III, n = 189) in the late phase. There were 255, 226, and 177 patients in the NE, EE, and LE groups, respectively. Adjuvant therapy was administered to 336 patients, no adjuvant therapy was given to 103 patients, and whether adjuvant therapy was administered was unknown in 219 patients. Of the patients who received adjuvant therapy, 213 received S-1 and 123 received anti-cancer drugs other than S-1 after surgery. During follow-up, 313 patients died and 345 were alive at the end-point of the study. Of the 313 patients who had died, 251 had died of the original cancer, 51 of comorbidities, and 11 of unknown causes.
Patient age and postoperative chemotherapy status are summarized in (Table 1). Significantly more patients with both stage II and stage III disease received S-1- after surgery in the late phase than in the early phase. There were no significant differences in the number of patients with stage II or III disease who received adjuvant therapy or in age distribution between the early and late phases.
Adjuvant treatment by age group is summarized in (Table 2). There were significantly more patients with stage II and III disease in the NE group than in the EE and LE groups (stage II: NE vs EE, p = 0.0024; NE vs LE, p < 0.0001; EE vs LE, p = 0.0009; stage III: NE vs EE, p < 0.0001; NE vs LE, p < 0.0001; EE vs LE, p < 0.0001).
Deaths by age group are summarized in (Table 3). Significantly more patients with stage II disease died of comorbidities in the LE group than in the NE or EE groups (NE vs EE, p = 0.0994; NE vs LE, p = 0.0001; EE vs LE, p = 0.0306). However, among stage III patients there were no significant differences in the number of deaths in each age group caused by comorbidities (NE vs EE, p = 0.8695; NE vs LE, p = 0.0508; EE vs LE, p = 0.0697).
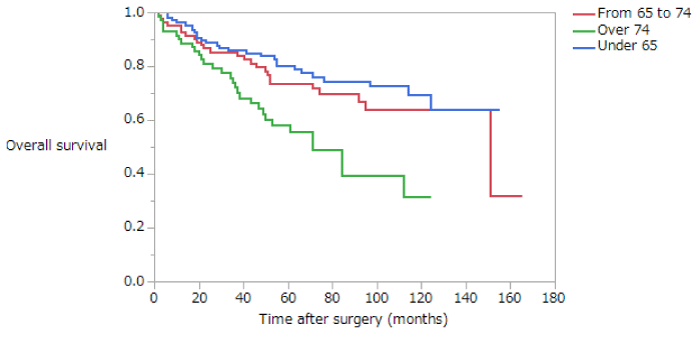
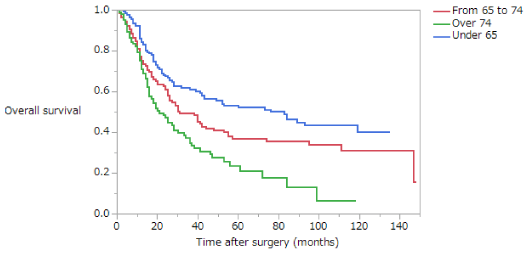
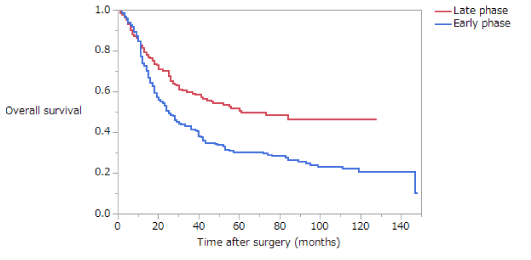
Overall survival (OS) is summarized in (Table 4). The 5-year survival rates of patients with stage II disease in the NE, EE, and LE groups were 80.4%, 74.5%, and 56.7%, respectively. The OS of patients with stage II disease was significantly lower in the LE group than in the NE or EE groups (NE vs EE, p = 0.3370; NE vs LE, p = 0.0002; EE vs LE, p = 0.0077) (Figure 1). The 5-year survival rates of patients with stage III disease in the NE, EE, and LE groups were 53.5%, 39.7%, and 20.8%, respectively. The OS of patients with stage III disease was significantly lower in the LE group than in the NE or EE groups, whereas the OS of the EE group was significantly lower than that of the NE group (NE vs EE, p = 0.0230; NE vs LE, p < 0.0001; EE vs LE, p = 0.0090) (Figure 2). The 5-year survival rate of patients with stage II disease was 72.9% in the early phase and 73.0% in the late phase; this difference is not significant (Figure 3). The 5-year survival rate of patients with stage III disease was 31.9% in the early phase and 51.8% in the late phase. The OS of patients with stage III disease was significantly higher in the late phase than in the early phase (p < 0.0001) (Figure 4); in both NE and EE groups, the OS was significantly higher in the late phase than in the early phase (p = 0.0082 and p = 0.0035, respectively). The 5-year survival rate of patients with stage II disease was 61.3% for those who had not received adjuvant therapy and 74.3% for those who had; this difference is not significant. However, in the late phase, OS was significantly higher in patients with stage II disease who had received adjuvant therapy than those who had not (p = 0.0117). The 5-year survival rate of patients with stage III disease was 26.5% for those who had not received adjuvant therapy and 49.2% for those who had; this difference is statistically, significant (p = 0.0007). In the late phase, OS was significantly higher in patients with stage III disease who had received adjuvant therapy than in those who had not (p = 0.0003). However, in the early phase there was no significant difference in OS between patients with stage III disease who had and had not received adjuvant therapy.
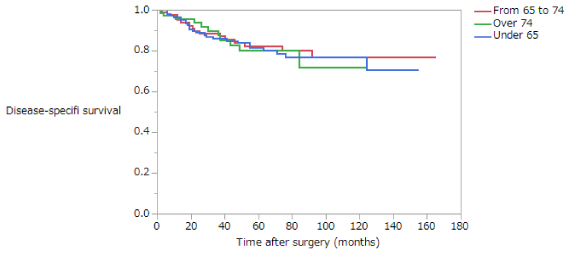
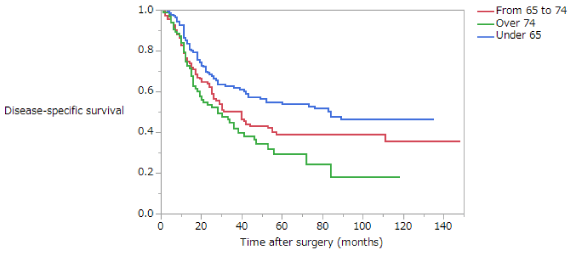
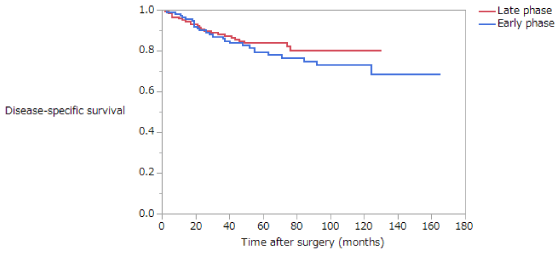
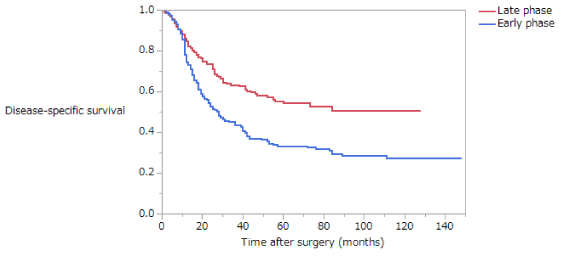
Disease-Specific Survival (DSS) is summarized in (Table 5). The 5-year survival rates of patients with stage II disease in the NE, EE, and LE groups were 82.6%, 83.5%, and 81.9%, respectively; these difference are not significant (Figure 5). The 5-year survival rates of patients with stage III disease in the NE, EE, and LE groups were 55.6%, 42.7%, and 27.0%, respectively. Among patients with stage III disease, DSS was significantly longer in the NE than in the EE or LE groups (NE vs EE, p = 0.0367; NE vs LE, p = 0.0004; EE vs LE, p = 0.1437) (Figure 6). The 5-year survival rate of patients with stage II disease was 81.2% in the early phase and 84.4% in the late phase; these difference are not significant (Figure 7). The 5-year survival rate of patients with stage III disease was 35.9% in the early phase and 56.0% in the late phase, the latter being significantly higher (p < 0.0001) (Figure 8). Moreover, in each age group, the DSS of patients with stage III disease was significantly longer in the late phase than in the early phase (NE, p = 0.0115; EE, p = 0.0052; LE, p = 0.039). The 5-year survival rate of patients with stage II disease was 85.4% in those who had not received adjuvant therapy and 77.8% in those who had. There were no significant differences in DSS of patients with stage II disease between those who had and had not received adjuvant therapy or in those in the early versus late phase. The 5-year survival rate of patients with stage III disease was 35.2% in those who had not received adjuvant therapy and 51.5% in those who had. DSS was significantly longer in patients who had received adjuvant therapy than in those who had not in all patients with stage III disease (p = 0.0283).
Discussion
In this study, adjuvant chemotherapy such as S-1 appeared to prolong the life expectancy of patients with stage II and III gastric cancer. However, the lives of patients in the LE group were not prolonged because of the low frequency with which they received adjuvant chemotherapy and their high comorbidity-related mortality.
Adjuvant chemotherapy is delivered with the intention of reducing the incidence of recurrence by controlling residual tumor cells following curative resection. Various regimens had been tested in numerous clinical trials in Japan without producing solid evidence in support of adjuvant chemotherapy until the ACTS-GC trial in 2006 showed that S-1 is effective [2,5].
The Japanese ACTS-GC trial demonstrated the efficacy of S-1 in patients with stage II and III gastric cancer after curative resection including D2 lymphadenectomy; S-1 improved the 3-year OS from 70.1% for surgery alone to 80.1% [2]. The S-1 group had a 5-year OS of 71.1%, compared with 61.1% in the surgery-alone group, corresponding to a 33% reduced risk of death. However, approximately 35% of patients still develop recurrence despite adjuvant S-1; subgroup analyses have suggested that S-1 is less efficacious for stage IIIB gastric cancer, in contrast to clear survival benefit demonstrated for stage II and stage IIIA disease to ACTS-GC [5]. Several chemotherapeutic agents, including cisplatin (CDDP) [18], irinotecan [19], taxanes (paclitaxel and docetaxel) [20,21], and oxaliplatin [22,] in combination with S-1 have demonstrated beneficial activity against gastric cancer and offer hope for improving patient outcomes. Several chemotherapy regimens, such as S-1 plus CDDP in the SPIRITS trial [23], have shown a remarkably high response rate. Accordingly, the Japanese Gastric Cancer Association Guideline recommends S-1 adjuvant chemotherapy for patients with stage II-III gastric cancer [6]. We now administer adjuvant S-1 after curative surgery for stages IIA, IIB, IIIA, IIIB, and IIIC gastric cancer according to the Japanese Classification, 14th Edition [17]. In this study, significantly more patients received S-1 after curative surgery for stage II and III cancer in the late phase than in the early phase, the results of the ACTS-GC trial having been released at the end of the early phase [2]. Although there was no significant difference in the proportion of patients receiving some form of adjuvant therapy between the early and late phases, both OS and DSS of patients with stage III disease were significantly better in the late phase than in the early phase. Patients with stage II and III disease who had received adjuvant therapy had a significantly higher OS than did those who had not in the late phase; however, their OS did not differ significantly from that of those in the early phase. In the late phase, patients with stage III disease who had received adjuvant therapy tended to have a better DSS than did those who had not. We consider that these differences in survival in the late phase between patients who had and had not received adjuvant therapy are attributable to the use of S-1 as adjuvant therapy in the late, but not the early, phase.
There was no significant difference in DSS by age distribution in patients with stage II disease; however, the number of comorbidity-related deaths was significantly higher in the LE than in the NE or EE groups. This resulted in LE group patients with stage II disease having a significantly lower OS than those in NE or EE group. LE group patients with stage III disease had significantly lower OS and DSS than did those in the NE or EE group, this result being considered attributable to the low frequency of administration of adjuvant therapy to the LE group. Among patients with stage III disease, OS and DSS were higher in the late phase than in the early phase. However, the OS of LE group patients with stage III disease did not differ significantly between the early and late phases.
Adjuvant chemotherapy after surgery should be given cautiously to LE patients with stage II disease because of their high frequency of comorbidity-related death. Hikage, et al. compared the feasibility, safety, and surgical outcomes of gastrectomy in patients with gastric cancer aged ≥ 85 years versus those of patients aged 75-84 years [24]. They concluded that chronological age alone is not a valid reason to avoid gastrectomy; rather, comprehensive assessment is necessary to determine the optimum treatment strategy for older patients with gastric cancer. Therefore, we consider it appropriate to administer adjuvant chemotherapy to LE patients with stage III gastric cancer who have undergone curative surgery, have no serious comorbidities, and have a good performance status. However, given that the upper age limit was 80 years in the ACTS-GC trial [2], adjuvant chemotherapy should be administered with great care.
Acknowledgment
We thank Doctor. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
- International agency for research on cancer/ WHO. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. https://bit.ly/31qXUi0
- Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357: 1810-1820. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17978289
- Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Validation of staging systems for gastric cancer. Gastric Cancer. 2008; 11: 111-118. https://bit.ly/2UrOSzZ
- Shirasaka T, Shimamato Y, Ohishima H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996; 7: 548-557. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8862723
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29: 4387-4393. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22010012
- Japanese gastric cancer association. Gastric cancer treatment guideline. Tokyo. Kanehara. 2018.
- United Nations. Department of economic and social affairs. World population prospects. 2015 revision. https://bit.ly/31tjpyT
- Ministry of health, labour and welfare. Abridged life tables for Japan. 2015.
- Statistics Bureau, Ministry of Internal Affairs and Communications. Population estimates by age (5-year age groups) and sex.
- Center for cancer control and information services. National cancer center. Japan. Incidence of stomach cancer (National Estimates).
- Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, et al. Comparison of surgical outcomes of gastric cancer elderly and middle-aged patients. Am J Surg. 2006; 191: 216-224. https://bit.ly/31zEHLe
- Orsenigo E, Tomajer V, Palo SD, Carlucci M, Vignali A, Tamburini A, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer. 2007; 10: 39-44. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17334717
- Passot G, Vaudoyer D, Messager M, Brudvik KW, Kim BJ, Mariette C, et al. Is extended lymphadenectomy needed for elderly patients with gastric adenocarcinoma. Ann Surg Oncol. 2016; 23: 2391-2397. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27169773
- Brenkman HJF, Goense L, Brosens LA, Haj Mohammad N, Vleggaar FP, Ruurda JP, et al. A high lymph node yield is associated with prolonged survival in elderly patients undergoing curative gastrectomy for cancer: a Dutch population-based cohort study. Ann Surg Oncol. 2017; 24: 2213-2223. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28247154
- Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. 2013; 37: 2891-2898. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24081528
- Straatman J, Van der Wielen N, Cuesta MA, de Lange de Klerk ES, van der Peet DL. Major abdominal surgery in octogenarians: should high age affect surgical decision-making. Am J Surg. 2016; 212: 889-895. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27270411
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14: 101-112. https://bit.ly/2UqsvLd
- Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011; 67: 1423-1428. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20809123
- Inokuchi M, Yamashita T, Yamada H, Kojima K, Ichikawa W, Nihei Z, et al. Phase I/II study of S-1 combined with irinotecan for metastatic advanced gastric cancer. Br J Cancer. 2006; 94: 1130-1135. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16570038
- Ueda Y, Yamaguchi H, Ichikawa D, Okamoto K, Otsuji E, Morii J, et al. Multicenter phase II study of weekly paclitaxel plus S-1 combination chemotherapy in patients with advanced gastric cancer. Gastric Cancer. 2010; 13: 149-154. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20820983
- Kanazawa Y, Kato S, Fujita I, Onodera H, Uchida E. Adjuvant chemotherapy with S-1 followed by docetaxel for gastric cancer and CY1P0 peritoneal metastasis after relatively curative surgery. J Nippon Med Sch. 2013; 80: 378-383. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24189356
- Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015; 26: 141-148. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25316259
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRIT trial): a phase III trial. Lancet Oncol. 2008; 9: 215-221. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18282805Hikage M, Tokunaga M, Makuuchi R, Irino T, Tanizawa Y, Bando E, et al. Surgical outcomes after gastrectomy in very elderly patients
- with gastric cancer. Surg Today. 2018; 48: 773-782. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29536199


Sign up for Article Alerts