Hepatoprotective Property of Dihydromyricetin against Tripterygium wilfordii Glycosides-Induced Liver Injury in Mice
Han Cheng1, Mei Li1, Jun Li1, Hui Chen1, Awais Ihsan3 and Xianju Huang1,2*
1College of Pharmacy, South-Central University for Nationalities, China
2National Demonstration Center for Experimental Ethnopharmacology Education, South-Central University for Nationalities, Wuhan P.R. China, 430074
3Department of Biosciences, COMSATS Institute of information Technology, Sahiwal, Pakistan
*Address for Correspondence: Xianju Huang, Dr. College of Pharmacy, South-Central University for Nationalities, Wu’han, Hubei, 430074, China; E-mail: xianju@mail.scuec.edu.com
Submitted: 10 September 2017; Approved: 23 October; Published: 25 October 2017
Citation this article: Cheng H, Li M, Li J, Chen H, Huang X, et al. Hepatoprotective Property of Dihydromyricetin against Tripterygium wilfordii Glycosides-Induced Liver Injury in Mice. Adv J Toxicol Curr Res. 2017;1(2): 056-062.
Copyright: © 2017 Huang X, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Tripterygium wilfordii Glycosides; Ampelopsis grossedentata; Dihydromyricetin; Hepatoxicity; Oxidative Stress
Download Fulltext PDF
Tripterygium wilfordii Glycosides (TG) tablet, a patented traditional Chinese medicine derived from Tripterygium wilfordii extracts, is limited by its narrow therapeutic window and severe toxicity. Dihydromyricetin (DMY), a flavonoid extracted from Ampelopsis grossedentata, may possess protective potential on drug-induced hepatic injury based on previous studies. Here, we analyzed the effect of DMY on TG-induced hepatotoxicity in mice. The TG-induced hepatotoxicity was demonstrated by the decreased body weight, increased liver index, altered hepatic histology, increased plasma levels of hepatic enzymes and hepatic expression of MicroRNA-122 in comparison with the treatment of water in both male and female mice. Pre-treatment with DMY for 8 days attenuated all aforementioned TG-induced toxic effects including oxidative stress in the liver. In conclusion, our data indicated that the treatment with DMY protected the liver from TG-induced toxicity. Therefore, it could serve as an antidote to attenuate the toxicity of TG-based chemotherapy.
Introduction
Drug-Induced Liver Injury (DILI) is a rare and often unpredictable adverse reaction to many drugs in common use. It represents a leading cause of acute liver failure in Western countries and one of the most common reasons for attrition during drug development and adoption of post marketing regulatory actions [1]. Herbal medicine has been used for thousands of years, particularly in East Asia [2]. The average one-year prevalence of use of herbal products in the United States and UK is approximately 25% and 41.1%, respectively [3,4]. They also lead to DILI frequently in clinic. Current principal strategies for treating different liver hepatic diseases contain pharmacotherapy, surgery, and liver transplantation. However, all the treatments mentioned above exhibit limited therapeutic benefits and are even associated with serious complications or toxicities, which is therefore prompt us to search for novel, alternative and effective treatments against DILI [5,6].
Ampelopsis grossedentata (Hand-Mazz.) W. T. Wang, also known as vine tea, an indigenous plant in South China, is widely distributed in several provinces in the southern region of the Yangtze River, such as Hubei, Fujian, Guangdong, Guangxi, Guizhou, Hunan, Jiangxi and Yunnan province in China. The tender stems and leaves of vine tea, have been widely utilized not only as a healthy tea, but also as a medicinal herb in Traditional Chinese Medicine (TCM) to cure disease both by internal and external administration [7]. As recorded in the Chinese Materia Medicai, vine tea can clear away heat, promotes dieresis and blood circulation, and removes channel obstructions. Indigenous people including the Tujia, Lahu, Yao, and Dong in China have used vine tea to treat hypertension and other diseases such as fever, icteric viral hepatitis, chronic pharyngitis and scabies for hundreds of years [8]. A growing body of researches suggested that the bioactivity of vine tea is due to various beneficial effects of Dihydromyricetin (DMY), also called ampelopsin. Previous studies suggested a strong intrinsic activity and efficacy of DMY as therapeutic component for various diseases, including alcohol use disorders and chronic pharyngitis [10]. It also showed pharmacological activities including hepatoprotective, antioxidative, anti-inflammatory and antihypertensive effects [11]. As the most abundant ingredient in vine tea, DMY was isolated from the tender stem and leaves of the Ampelopsis grossedentata species. It’s reported that the content of DMY in the mother plants could be as high as 39.4% (w/w) [9].
Tripterygium wilfordii Glycosides (TG) tablets, a patented traditional medicines derived from Tripterygium wilfordii (TW) extracts, is limited by its narrow therapeutic window and severe toxicity on several organs including liver and kidney [12,13]. In clinical application of TG for patients with inflammation and autoimmune diseases, TG is recommended for oral administration under the guidance of a doctor. To the best of our knowledge, no previous investigation of DMY on TG-induced hepatic injury has been reported.
The present study was thus conducted to estimate the hepatoprotective property of TMY on DILI induced by TG. We have therefore examined the ameliorative potential of DMY against TG tablets induced hepatotoxicity in mice, which may provide a scientific basis for the clinic use of A. grossedentata in the future.
Methods
Chemicals and reagents
TG tablets were purchased from Feiyuan pharmaceutical Co. Ltd. (Huangshi, Hubei, China). The pulverized powder of TG tablets were dissolved in distilled water and promoted by ultrasound to make suspension.
Preparation of DMY from vine tea
The dried leaves of Ampelopsis grossedentata were purchased in replicate packages from tea stores in Enshi, Hubei Province in China. The leaves were authenticated by Dr. Jun Li, School of Pharmacy, South-central University for Nationalities, China. The voucher specimen (No. S20140710, respectively) has been deposited at the Herbarium situated in College of Pharmaceutical Sciences.
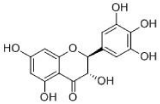
DMY (to see in Figure 1) was prepared in our lab. Briefly, the plants (200 g) were crushed into small pieces. The mixture was refluxed with water (1:10, w/v) for 2 h. The filtrates were collected and the residues were then refluxed in water (1:10, w/v) for 1.5 h. Two batches of filtrates were combined. Afterwards, the concentrated extract was dried by vacuum concentration to obtain the DMY extract with a yield of 25.6% (w/w, dried extract/crude herb).
Animals and Administration
KM mice (18-22 g) were obtained from the Center of Laboratory Animals of Hubei Province (Wuhan, P.R.China). Animals were maintained in a room with controlled temperature (22 ± 2 °C) for 12-h light/dark cycle with free access to food and water. Twelve hours before each experiment, animals received only water in order to avoid food interference with substance absorption. All the animal protocols conformed to the National Institutes of Health (NIH) guidelines and were approved by the Ethical Committee in South-Central University for Nationalities (No. SYXK2015-0008).
The dosage of TG and DMY was selected according to the clinical dose and our preliminary experiment. 50 mice were randomly and equally assigned to 5 groups (5 males and 5 females per group). The first group (control) received sterile water, given orally every day, next four groups received oral administration of DMY at dose of 0, 50, 100 and 250 mg/kg, respectively for 8 days. Then each treated group received additional administration of TG (270 mg/kg) orally for 3 days. Water and food were freely available to the animals.
During this period, the general behavior and number of survivors were observed daily thereafter until day 15. The body weight of the animals were recorded in every day. The food as well as water intake were assessed. At the end, all mice were sacrificed and their livers were carefully dissected and weighed to calculate the organ index. The liver index was calculated as liver weight to body weight ratio. Then the livers were separated for histopathological analysis or measurements of MicroRNA-122 (miR-122) and oxidative indexes, respectively. Blood samples were collected for serum biochemistry examination.
Histopathology
The liver samples from different mice in each group were collected and fixed in 10% neutral-buffered formalin and mounted on slides for histopathological examination. Histopathological examination was conducted by using routine paraffin embedding technique. Sections of 4 μm thickness stained with Hematoxylin and Eosin (H&E) were examined and compared among treatment groups under an optical microscope (Olympus BX 41) for morphological alterations [14].
Clinical biochemistry and measurements of oxidative stress indexes
The serum biochemistry analysis for Alkline Phosphatase (AKP), Lactic Dehydrogenase (LDH), Alanine Transaminase (ALT), and Aspartate Transaminase (AST) were measured by using commercial kits from Nanjing Jiancheng Bioengineering Institute according to the manufacturer’s directions.
The liver tissue samples were homogenized with NS (1:9) and centrifuged at 1500 x rpm for 5 min at 4. The supernatant was collected and diluted to 1% with normal saline. Then 1% supernatant was splitted into four parts. Three was subjected to reduced Glutathione (GSH), Catalase (CAT), Superoxide Dismutase (SOD) and Malondialdehyde (MDA) analysis, respectively. The remaining one was subjected to the measurement of tissue protein levels using commercial kits from Jiancheng-Bioeng Institute. All experimental results were normalized according to total protein levels of the samples.
Reverse transcription (RT) and real-time PCR for miRNA and mRNA quantification
Quantitative Real-time PCR was used to determine the gene expression of miRNA-122 in the liver of mice. Briefly, total small RNA was extracted from livers using the RNAsio reagent (Qiagen, Valencia, CA). For mRNA quantification, cDNA was synthesized from 500 ng of total RNA using the PrimeScriptTM RT reagent Kit (TaKaRa) according to the manufacturer's instructions and stored at −20°C. Reactions were performed in a 20 μL volume according to the thermal cycle manufacturer's protocol (Rox) using an Real-Time PCR system (Applied Biosystems) under the following conditions: 30s at 95°C followed by 40 cycles of 5 s at 95°C, and 40s at 60 °C [15]. The primer sequences used for amplification of target genes are listed as below.
Statistical analysis
Data were presented as means ± SD. Multiple comparisons were analyzed by one-way ANOVA using Least Significant Different (LSD) when equal variance was assumed, and Games-Howell test was used when equal variance was not assumed. Differences were considered statistically significant when P < 0.05.
Results
DMY (50-250 mg/kg) could ameliorate the liver damage induced by TG
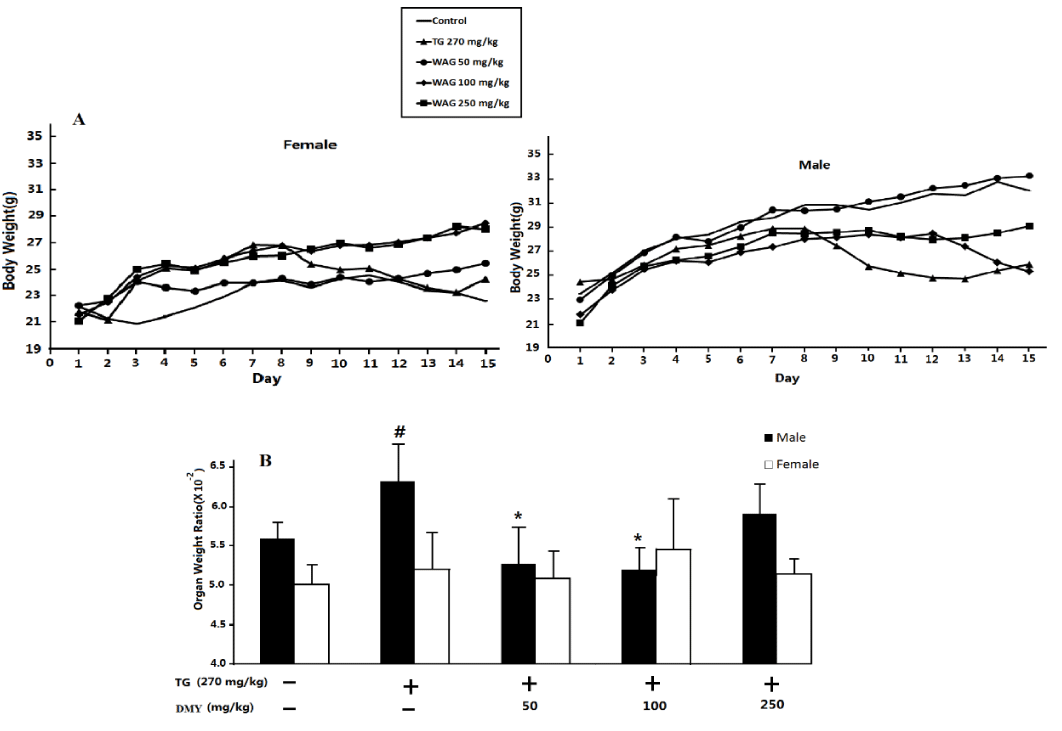
All the male and female mice were survived but the mice received 270 mg TG/kg were weak and emaciated. DMY (50-250 mg/kg) could ameliorate the clinical symptoms to some extent. As shown in figure 2A, the body weight of control group increased steadily till the end the experiment. Administration of TG led to severe decrease of body weight in comparison with that of the controls for both male and female mice (P < 0.05), and then increased slowly. DMY (50-250 mg/kg) could ameliorate the decrease of body weight induced by TG treatment in either male or female groups to some extent (P < 0.05).
At the same time, the liver index in male mice receiving 270 mg TG/kg was significantly higher than that of the control mice. DMY (50, 100mg/kg) attenuated the increase of liver index in TG-treated mice to some extent, however, the liver index of female mice don’t have obvious changes. (To see in figure 2B).
Clinical pathology
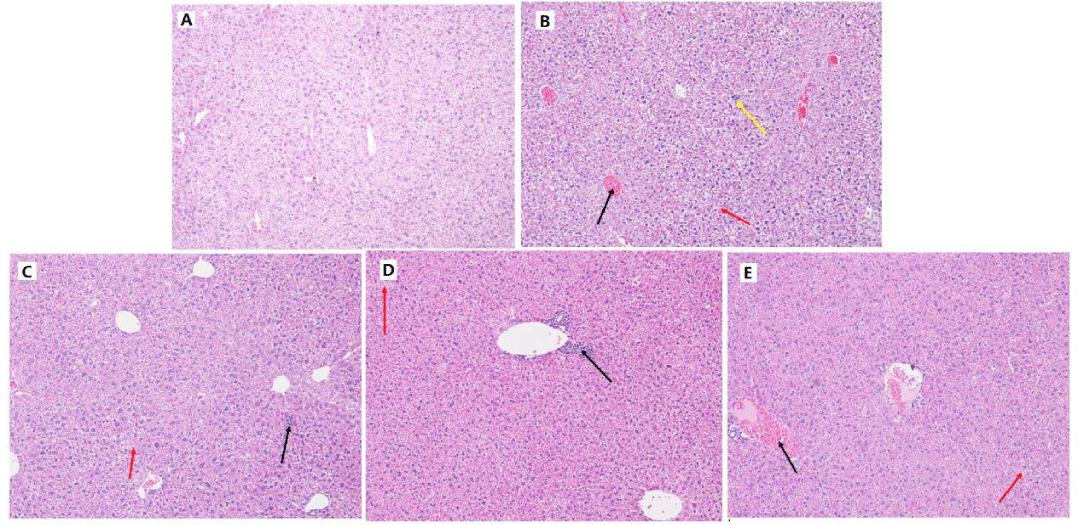
As shown in figure 3, distinct overall structure abnormality of the liver was observed histologically after TG treatment (figure 3B), including the congestion in central vein (indicated as the black arrow), extensive oedema and severe vacuolar degeneration of hepatocyte (as shown in the red arrows) and serious inflammatory cell infiltration of liver parenchyma (as shown in the yellow arrow). DMY (50-250 mg/kg) could all reduce the pathological damage of liver to some extent (figure 3C-E). The liver injury of DMY treated mice was observed as individual inflammatory lesions in the portal areas, mild oedema and vacuolar degeneration of hepatocyte.
Clinical biochemistry
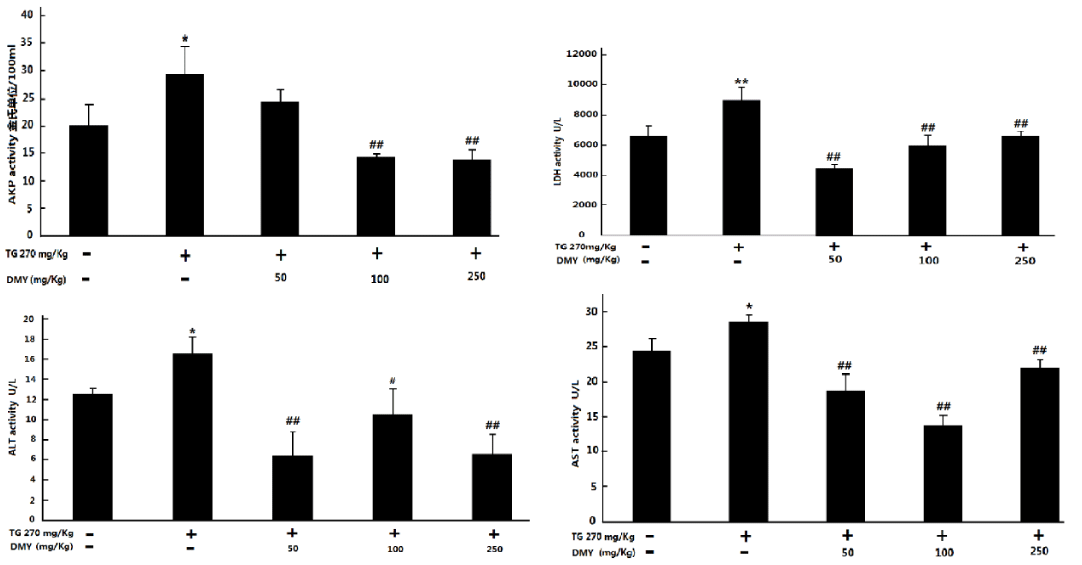
The release of hepatic enzymes in the blood reflects the liver injury. As shown in figure 4, the serum levels of ALT, AST, ALP and LDH were increased in mice treated with TG. However, DMY (50-250 mg/kg) treatment could downregulate the above-mentioned indexes to some extend (T Influence of DMY on TG-induced miR-122 of KM mice.
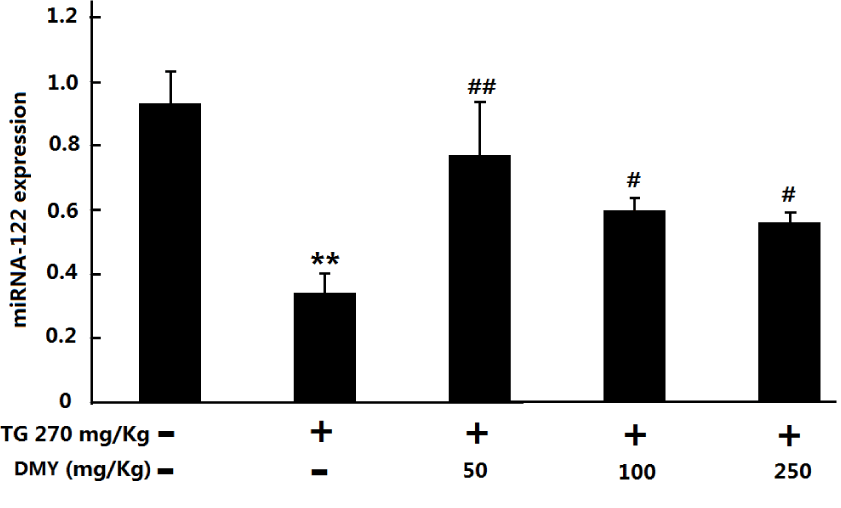
Level of miR-122 was found to have an important role in liver function and is involved in multiple metabolic processes, including cholesterol biosynthesis [16], Fatty acid synthesis, and β-oxidation [17]. Therefore, we analyzed the miR-122 expression levels in the liver of those mice without haematolysis as indicated in figure 5 TG could induce obvious decrease of gene levels of miR-122 in the mouse liver. On the other hand, significant increase of transcription levels of miR-122 transcripts occurred in the liver of those mice pre-treated with different doses of DMY compared with that in the TG group.
Parameters of oxidative stress
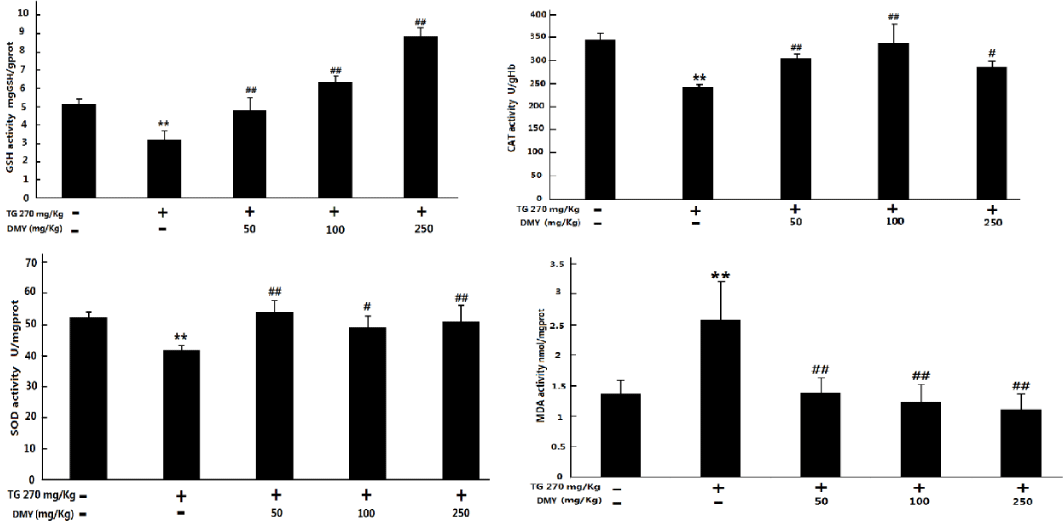
As shown in figure 6, TG treatment could lead to obvious increase of MDA as well as decrease of SOD, CAT and GSH levels in the mouse liver (P < 0.05 compared with control group). The pre-treatment with DMY (50 -250 mg/kg) could attenuate the above changes to some extent (P < 0.05 vs TG group).
Discussion
Hepatotoxicity of TG was observed in the present study as shown by decrease of body weight, increase of liver index and histological changes of liver tissue compared with controls. The reduction of body weight illustrated that the anabolism of proteins and the energy metabolism in the TG-treated mice were seriously damaged, and reflected the hepatotoxicity of TG from another perspective. An increase in the liver index should be considered as a liver specific change, which was further supported by the noticeable effect on the histological evaluation conducted in the liver samples of the mice treated with TG. Moreover, administration of TG (270 mg/kg) induced hepatic damage as indicated by serum marker enzymes ALT, AST, ALP and LDH as well as miR-122 in liver. DMY treatment clearly protected against TG-induced hepatotoxicity as shown by the restoration of these injury biomarkers in serum and liver which indicate hepatoprotective effect.
Serum marker enzymes as well as miR-122 are attractive and selective therapeutic target for the treatment of liver diseases [18,19]. Drug induced hepatotoxicity involves cellular injury as a consequence of which the cytosolic enzymes (AST, ALT, ALP and LDH) leak out leading to their raised levels in the blood [20]. Clinically, the norm alization of ALT is taken as the main efficacy assessment of liver function. However, the level of liver damage does not correlate with ALT alterations because ALT distributes widely in extrahepatic tissues. In contrast to those serum marker enzymes, miR-122 is a conserved liver-specific miRNA that accounts for 70% of the total miRNA population [21-25] and plays important roles in liver physiology, such as lipid metabolism [25,26] diseases in hepatic virus C (HCV) infection [27] and hepatocellular carcinoma (HCC) [26]. miR-122 levels are reduced in Hepatocellular Carcinoma (HCC) compared with normal liver cells, and low miR-122 expression correlates with poor prognoses [28,29]. Thus, miR-122 is a key factor and therapeutic target in liver disease. The miR-122 levels are widely expected to be a marker of prognostic and diagnostic liver disease [30].
Induction of oxidative stress due to excessive formation of Reactive Oxygen Species (ROS) which causes lipid peroxidation of the cellular membrane. It has been reported that the toxicity of TW related preparations may result from over-accumulation of ROS and oxidative stress in hepatocytes, leading to cell dysfunction and even apoptosis or necrosis [31,32]. The present study revealed that TG induced oxidative stress in the liver as evident from the increase of MDA level, decreases of GSH level and SOD activity. MDA is one of the major end products of Lipid Peroxidation (LPO), and is commonly used as an indicator for liver tissue damage. The content of MDA is an index of intensified peroxidation process. SOD protects liver against spontaneous oxygen toxicity and lipid peroxidation. GSH plays a vital role in protecting cells against oxidative injury. SOD and GSH play important roles in oxidation, and both together can effectively prevent tissue damage through oxidation. In our study we have shown that DMY protects against TG hepatotoxicity by preventing the induction of oxidative stress and enhancing the hepatic antioxidant defenses involving SOD, MDA and GSH. Our study concludes that TG induces oxidative stress in the liver and the DMY effectively prevents TG hepatotoxicity through boosting the antioxidant capacity of the liver. The bioactive antioxidant principles present in the extract could be responsible for the hepatoprotective effect.
The present study suggested that DMY possessed significant Hepatoprotective activity by decreasing the levels of AKP, AST, ALT and LDH, increasing the activities of liver antioxidant enzymes and miR-122, thus alleviating liver tissue injuries, the application of DMY may help reduce the hepatotoxicity of TG and thus may deduce new applications in clinical.
Overall, our study concludes that TG induces oxidative stress in the liver and the water extract of DMY, a major flavonoid isolated from A. grossedentata, effectively prevents TG hepatotoxicity by scavenging free radicals, improving the antioxidant capacity of liver cells and inhibiting lipid peroxidation in vivo. The bioactive antioxidant principles present the extract can improve the liver enzymes, lipid metabolism, and biochemical parameters in DILI, could be responsible for the hepatoprotective effect. It is expected that our findings should be highly useful in helping determine the interactions between TG hepatotoxicity and the DMY-induced amelioration involved in many biological processes and pathways. Furthermore, we believe that this study provides novel insight into the strategies of the detoxication of TG-induced hepatotoxicity.
The use of herbals dates back as far as 2100 BC in TCM. The general use of herbal drug show ever raises of medical issues regarding their adverse effects, particularly hepatotoxicity [33]. One group in China indicated that herbal drugs caused 24.2% of cases of DILI [34]. Therefore, there is strong demand for remedies to attenuate TCM-associated hepatotoxicity. The key problem with toxic Chinese herbs in clinical applications is how to find the most effective method to reduce toxicity. Most Chinese medicine are mixtures of extracts or preparation of several different herbs of which one or two are considered the pharmacologically active King herbs [35]. The present study suggested that the application of DMY may help reduce the hepatotoxicity of TG and thus indicated that usage compatibility with TW and A. grossedentata was reasonable in clinical settings. The folk medicine of A. grossedentata therefore could be a potential source of a therapeutics use as an adjunct in rheumatoid arthritis chemotherapy in order to reduce the toxic side effects of TW or TG. To the best of our knowledge, this is the first report to describe the mechanism of reducing hepatotoxicity with the use of DMY from clinical experie.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81102897 and 81374064).nce.
- Kaplowitz N, Deleve LD. Drug-induced liver disease. Academic press. 2013; 3: 677-685. https://goo.gl/qekHb7
- Ye XF, He J. The bright future of Chinese herbal medicine: only after a twisty road. Contemp Clin Trials. 2010; 31: 508-509. https://goo.gl/AG3gm2
- Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004; 116: 478-485. https://goo.gl/VY7chW
- Posadzki P, Watson LK, Alotaibi A, Ernst E. Prevalence of use of complementary and alternative medicine (CAM) by patients/consumers in the UK: systematic review of surveys.Clin Med. 2013; 13: 126-131. https://goo.gl/VmXNPc
- Bishayee A, Darvesh AS, Politis T McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010; 30: 1103-1114. https://goo.gl/97fPo2
- Mihailovic V, Mihailovic M, Uskokovic A, Arambasic J, Misic D, Stankovic V, et al. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in mice. Food Chem Toxicol. 2013; 52: 83-90. https://goo.gl/mcjKqh
- Tong Q, Hou X L, Fang JG, Wang W, Xiong W, Liu X et al. Determination of dihydromyricetin in rat plasma by LC–MS/MS and its application to a pharmacokinetic study. J Pharm Biomed Anal.2015; 114: 455-461. https://goo.gl/RZfFbr
- Hou X, Tong Q, Wang W, Xiong W, Shi C, Fang J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life sci. 2015; 130: 38-46. https://goo.gl/vXPm14
- Gao W, Lee SU, Li J, Jin Woo Lee. Development of improved process with treatment of cellulase for isolation of ampelopsin from dried fruits of ampelopsis grossedentata. BioResources.2016; 11: 2712-2722. https://goo.gl/yExZYY
- Kou X, Chen N Pharmacological potential of ampelopsin in Rattan tea. Food Sci Hum Welln. 2012; 1: 14-18. https://goo.gl/rRom5Y
- Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang C, et al. Dihydromyricetin reduced bcl-2 expression via p53 in human hepatoma hepg2 cells. PLoS One. 2013; 8: 1-7. https://goo.gl/obMPBX
- Li XX, Du FY, Liu HX, Ji JB, Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J Ethnopharmacol. 2015; 162: 238-243. https://goo.gl/S6YPSg
- Peng W, Qiu XQ, Shu ZH, Liu QC, Hu MB, Han T Peng W, Qiu XQ, Shu ZH, Liu QC, Hu MB, Han T et al. Hepatoprotective activity of total iridoid glycosides isolated from Paederia scandens (lour.) Merr. Var. tomentosa. J Ethnopharmacol. 2015; 174: 317-321. https://goo.gl/i9LJkp
- Ge YB, Jiang Y, Zhou H, Zheng M, Li J, Huang XJ et al. Antitoxic effect of Veratrillabaillonii on the acute toxicity in mice induced by Aconitum brachypodum, one of the genus Aconitum. J Ethnopharmacol. 2016; 179: 27-37. https://goo.gl/YiEexn
- Yu Y, Yi XJ, Mei ZY, Li J, Huang XJ, Yang GZ. The water extract of Veratrilla baillonii could attenuate the subacute toxicity induced by Aconitum brachypodum. Phytomedicin. 2016; 23: 1591-1598. https://goo.gl/nduMmP
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005; 438: 685-689. https://goo.gl/GSjthW
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting.Cell Metab. 2006; 3: 87-98. https://goo.gl/au53T7
- Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN. Antihepatotoxic properties of picroliv: an active fraction from rhizomes of Picrorhiza Kurroa.J Ethnopharmacol. 1991; 34: 61-68. https://goo.gl/RguBtc
- Lewis AP, Jopling CL. Regulation and biological function of the liver-specific miR-122. Biochem Soc Trans. 2010;38: 1553-1557. https://goo.gl/1aBCA6
- Senthilkumar S, Devaki T, Manohar BM, Babu MS. Effect of squalene on cyclophosphamide-induced toxicity. Clin Chim Acta. 2006; 364: 335-342. https://goo.gl/UE4iBD
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006; 125: 1111-1124. https://goo.gl/jrJ3VW
- Chang J1, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liverspecific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1 miR-122, a mammalian liverspecific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004; 1: 106-113. https://goo.gl/a5k8cs
- Jopling C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012; 9: 137-142. https://goo.gl/ZQQKjQ
- Li ZY, Xi Y, Zhu WN, Zeng C, Zhang ZQ, Guo ZC, et al. Positive regulation of hepatic miR-122 expression by HNF4a. J Hepatol. 2011; 55: 602-611. https://goo.gl/fVkFAe
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse, Curr Biol. 2002; 12: 735-739. https://goo.gl/w8KGXm
- Jianhua Gao, Benguo Liu, Zhengxiang Ning, Ruixiang Zhao, Aiyuan Zhang, Qiong Wu. Characterization and antioxidant activity of flavonoid-rich extracts from leaves of Ampelopsis grossedentata. J Food Biochem. 2009; 33: 808-820. https://goo.gl/aG2hT8
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science. 2005; 309: 1577-1581. https://goo.gl/6Mo7yo
- Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties.Oncogene. 2009; 28: 3526–3536. https://goo.gl/L8ZKxj
- Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006; 99: 671-678. https://goo.gl/UuUUj3
- Yamada H, Ohashi K, Suzuki K, Munetsuna E, Ando Y, Yamazaki M, et al. Longitudinal study of circulatingmiR-122 in a rat model of non-alcoholic fatty liver disease. Clinica Chimica Acta. 2015; 446: 267-271. https://goo.gl/HCW1RM
- Xue M, Jiang ZZ, Wu T, Li J, Zhang L, Zhao Y, et al. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine. 2012; 19: 998-1006. https://goo.gl/fE6oDj
- Hu J, Yu Q, Zhao F, Ji J, Jiang Z, Chen X, et al. Protection of Quercetin against Triptolide-induced apoptosis by suppressing oxidative stress in rat Leydig cells. Chem-Biol Interaction. 2015; 240: 38-46. https://goo.gl/C7sX94
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety.Front Pharmacol. 2013; 4: 177. https://goo.gl/rdetDg
- Li B, Wang Z, Fang JJ, Xu CY, Chen WX. Evaluation of prognostic markers in severe drug- induced liver disease. World J Gastroenterol. 2007; 13: 628-632. https://goo.gl/55uvLG
- Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005; 4: 489-499. https://goo.gl/nV1EiL

Sign up for Article Alerts