Urinary 8-Hydroxydeoxyguanosine and Biochemical Alterations as Biomarkers for Occupational Health Exposure to Pesticides and Fertilizers in Egypt
Khaled Yassin Abdel Halim1*, Nabila Mhoumed Said Bakry2 and Marwa Adel Abdel Wahab El Meseiry1
1Mammalian & Aquatic Toxicology Department, Central Agricultural Pesticides Laboratory (CAPL), ARC, 12618, Dokki, Egypt
2Pesticide Chemistry and Technology Department, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
*Address for Correspondence: Khaled Y Abdel Halim, Mammalian & Aquatic Toxicology Department, Central Agricultural Pesticides Lab. (CAPL), ARC, Egypt, Tel: +202-023-760-2209; E-mail: Khaled_yassen68@yahoo.com
Submitted: 22 April 2018; Approved: 30 September 2019; Published: 03 October 2019
Citation this article: Abdel Halim KY, Said Bakry NM, Wahab El Meseiry MAA. Urinary 8-Hydroxydeoxyguanosine and Biochemical Alterations as Biomarkers for Occupational Health Exposure to Pesticides and Fertilizers in Egypt. Adv J Toxicol Curr Res. 2019;3(1): 006-0014.
Copyright: © 2019 Agoro ES, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: 8-hydroxydeoxyguanosine; Urine; Pesticides; Fertilizers; Biochemical parameters; Occupational exposure
Download Fulltext PDF
The study was planned to assess the correlation between occupational exposure to pesticides and fertilizers and level of Urinary 8-Hydroxydeoxyguanosine (8-OHdG) and other biochemical parameters among workers of two companies (Kafr El-Zayat Company for pesticides and El-Malyia Company for fertilizers and chemicals) in Kafr El-Zayat district, Egypt. Nineteen participants from pesticides factory (1st group), 17 participants from fertilizers factory (2nd group) and 8 healthy persons were selected from rural region for the study. 8-OHdG levels of pesticide's exposed workers revealed the mean value 10.29 ± 1.60 ng/mg creatinine, but the 2nd group exhibited the mean value 12.47 ± 2.61 ng/mg creatinine compared with reference group (4.58± 1.03 ng/mg creatinine). The urea level revealed mean values 8.39 ± 0.84 and 13.89 ± 1.63 mg/dl for the 1st and 2nd group, respectively, compared with control group which did not exceed 8.37 ± 1.70 mg/dl. No significant difference obtained in case of uric acid and bilirubin assays, but creatinine levels exhibited lower values than those of control subjects. The correlations between biochemical variables and personal characteristics displayed positive concept with occupational lifespan, exposure period and smoking habits. These findings indicate that 8-OHdG and kidney function measurements may be good and simple biomarkers for assessing occupational exposure to pesticides and fertilizers.
Introduction
Occupational exposure to either pesticides or fertilizers occurs directly during manufacture of the products, transport and storage as well as during preparation, and spreading by the user [1,2]. Chemical inhalation mainly occurs during preparation in manufacturing plants. Occupational exposure of workers might be the most significant and has been extensively studied [3,4]. Concerning chemical group, pesticides are chemicals used in agriculture to control pests, and most of them are mixtures of several agents. However, fertilizers in concern are sulfur, nitrate, superphosphate, and acids, where the workers are exposed at the workplace by inhalation. Some data were recorded such as higher blood and urinary cadmium levels of 7.8 and 10 times, respectively [5]. The health hazards of workers who are exposed to risk factors have highlighted the need to develop a model study of the future of occupational health [6]. In regard this finding, pesticides and some fertilizer's by-products are associated with the increase of various types of cancer at specific sites such as skin, lung, prostate and brain tumors, non-Hodgkin lymphoma, Hodgkin's disease [7,8], leukemia [9], multiple myeloma [10], cancers of the immune, nervous, reproductive and hematological systems [11,12] and lipid discords [13,14]. On the other hand, many studies have pointed to the action of these chemicals as inducers of Chromosomal Aberration (CA) [15,16] and the formation of Micronuclei (MN) [17]. Also exposure to fertilizers is most commonly associated with contact dermatitis [18].
Many investigators demonstrated that, pesticides and heavy metals are capable of generating Reactive Oxygen Species (ROS) or Nitrogen Species (RNS) which cause oxidative damage to biomolecules. DNA is probably the most biologically significant target of oxidative attack. Among numerous types of oxidative DNA damage, the formation of 8-hydroxydeoxyguanosine (8-OHdG) which considered the major product of DNA oxidation and a sensitive biomarker of oxidative stress [19,20]. It is an adduct formed as a result of the reaction between free radicals and DNA and excreted in urine without further metabolism. Concentrations of 8-OHdG within a cell are a measurement of oxidative stress. As reviewed by [21], increased levels of 8-OHdG are frequently found during carcinogenesis. For human studies, several investigations demonstrated the association between workers exposure to chemicals and DNA damage. For example, workers who exposed to organophosphorus pesticides (applicators and farmworkers) showed 8-OHdG levels (8.5 and 3.4 times) greater than control [22]. Similar pattern was noted for pesticide sprayers and farmworkers in India, where urinary levels of 8-OHdG were found to be significantly greater than control group [20]. Another investigation by [23] noted that, paints workers exposed to ethylbenzene observed 8-OHdG levels greater than non-exposed group. On the other hand, prolonged exposure to multiple pesticides affects the liver, kidney, and physiological status [24]. Moreover, workers occupationally exposed to pesticides and heavy metals with high levels may alter creatinine, urea, uric acid and other parameters compared with non-exposed groups [25,26]. Thus, the study was done to evaluate the levels of 8-OHdG and some biochemical parameters in urinary samples of two exposed pesticides and fertilizers groups in Kafr El-Zayat region as biomarkers for occupational health status.
Material and Methods
Study design and personal information
This study was performed in two factories in Kafr El-Zayat district, El-Gharbia governorate, Egypt. It is one of the main industrial regions because it includes factory for the pesticides (KZ) and factory for fertilizers production (El-Malyia Company). Pesticides factory employs 800 workers in operation, formulation, packing, storage and commercial functions. Also, another group (50 persons) maintains official works. The factory records the highest level of pesticide's production in Egypt e.g. fentrithion, diazinon, chlorpyrifos, imidacloprid, methomyl, mineral oils, copper oxychloride, carbendazim, thiram, glyphosate, thiobencarb and bispyribac-sodium. Also, it maintains the production of sulphur and foliar fertilizers. On the other hand, El-Malyia Company employs 1.700 persons: 1.600 of them work in operation, formulation and commercial functions, as well as 100 persons are employed for office sector. The factory is considered the first plant for production of phosphoric acid, sulphric acid, superphosphate, potash minerals and sulphur. The factories were established about 50 year ago and have become old and less well maintained. Moreover, bad hygiene's were observed concerning production chambers and the frameworks remain unchanged.
The selected groups were 19 participants from Kafr El-Zayat Company for Pesticides Production and 17 participants from El-Malyia Company for Fertilizers and Chemicals. On the other hand, subjects in the control group consisted of 8 healthy men, who were not currently occupationally exposed to pesticides. They were randomly recruited in the rural site which is about 20 km from Kafr El-Zayat region. The subjects provided information through questionnaires regarding the sociodemographic characteristics, occupational activities, smoking habits, clinical characteristics and health status. Ethical report; IORG#..IORG0008812 was provided by Ethical Committee of Medical Research Institution, Alexandria University, Egypt.
Urine sample collection
Urine samples of all subjects were collected in sterile plastic tubes, marked and stored at -20°C till analyzed. Other samples were analyzed directly for some biochemical parameters e.g. creatinine, urea, uric acid and bilirubin.
8-OHdG determination
Each sample was brought to room temperature (25°C) and centrifuged at 2000 rpm for 5 min. The concentration of 8-OHdG in supernatant fraction was analyzed using Enzyme Linked Immunosorbent Assay (8-OHdG competitive ELISA kit-E0031 Ra, China). It is a competitive enzyme immunoassay developed for rapid detection and quantitation of 8-OHdG in urine, serum or other cell and tissue DNA samples. The quantity of 8-OHdG in the unknown samples is determined by comparing its absorbance with that of a known 8-OHdG standard curve. The kit has an 8-OHdG detection sensitive setting as 0.027 ng/ml. It provides sufficient reagents to perform up to 96 assays including standard curve and unknown samples. The oxidative DNA damage ELISA kit is a competitive technique for 8-OHdG measurement. The samples and 8-OHdG standards are first added to an 8-OHdG/BSA conjugate per absorbed 8-OHdG ELISA plate. After a brief incubation, an anti-8-OHdG monoclonal antibody is added, followed by an HRP conjugated secondary antibody. Levels of 8-OHdG in the unknown samples are determined by comparison with predetermined 8-OHdG standard curve.
Biochemical analysis
The following biochemical parameters were measured to study the effect of chemical exposure on the health status of workers compared with non-exposed group.
Uric acid: Uric acid level was estimated colorimetrically using SPINREACT diagnostic kit (Gerona, Spain) according to the procedure given in the kit protocol.
Urea: Urea level in urine samples was estimated colorimetrically using Diamond diagnostic kit (30175 Hannover, Germany). The mechanism depends on its hydrolysis to carbon dioxide and ammonia (NH4+) which reacts with salicylate and hypochlorite to form green indophenol. The developed color was measured at 578 nm against blank.
Creatinine: The level of creatinine in urine samples was used as an indicative of renal insufficiency. It is assayed according to procedure of Diamond diagnostic kit (30175 Hannover, Germany). The measurement was done at 492 nm against the blank and the value was expressed as mg/dl.
Bilirubin: Total bilirubin in urine samples was estimated colorimetrically using BIOMED diagnostic kit (EGY-CHEM for Lab. Technology, Egypt) according to the procedure given in the kit protocol. The measurement was done at 578 nm against the blank and the values were expressed as mg/dl.
Statistical Analysis
The measured variables were expressed as mean ± SE. To establish differences between variables, Analysis Of Variance (ANOVA) was used. The degree of association between variables was evaluated based on Pearson's or Spearman's correlation Coefficient performing with MSTAT-C2.1 program. A simple linear model was used to compare the measured parameters with personal characteristics of studied participants. All data were processed by Microsoft Excel (Microsoft 2000) and statistical analysis was conducted using the program of SAS Release 6.12 [27].
Results
This work demonstrated the impact of occupational exposure concerning workers groups from two remarkable plants in pesticides and fertilizers production in Egypt. In addition, the reference group (rural subjects) was employed as non-smoking, official staff and healthy, especially hepatitis status. Table 1 represents the characteristics of studied participants. The mean ( ± SD) age of the subjects was as follows: 41.30 ± 3.07, 36.96 ± 10.76 and 21.74 ± 13.83 yr for KZ Co., El-Malyia Co. and control group (P < 0.05), respectively. All exposed workers were employed for age categories as follows: 33-36; 37-40; 41-44; 45-48; 49-52; 53-56 and 57-60 yr (P < 0.005), respectively. Concerning exposure frequency, the workers were classified into three categories as follows: 0-10, 11-20 and 21-30 yr with mean values of 8.5 ± 1.40, 15.7 ± 2.80 and 26.1 ± 2.90 yr (P < 0.05), respectively. Therefore, exposure duration was recorded according to the following categories: 1, 2-4, 5 and 6 day/week; 4.35 ± 1.07, 2.17 ± 0.60, 26.09 ± 4.30 and 45.65 ± 18.14% (P < 0.05), respectively. Other jobs or activities of industrial worker's groups demonstrated the following categories; industrial only (n = 29), pesticides sprayer (n = 13), commercial (n = 4) and farmer (n = 4) with percentages; 43.48 ± 16.6, 23.91 ± 2.77, 2.17 ± 1.60, 8.70 ± 7.99% (P < 0.05), respectively. The awareness and use of protective clothing during factory working time was assessed, the data were 78.26 ± 19.98% for non-aware and 21.74 ± 9.99% for aware subjects. The health status of studied participants was investigated through cases of hepatitis, diabetes, heart diseases and others. Regarding hepatitis C, 45.65 ± 9.22% of the subjects were positive HCV, while 32.61 ± 7.69% were negative HCV (P < 0.05). On the other hand, 13.04 ± 6.60% of the participants were record diabetic, and 65.22 ± 10.37% were not (P < 0.05). Heart diseases were recorded only for 13.04 ± 9.35% of participants, while 65.22 ± 22.55% were healthy (P < 0.05). Regarding smoking habits, 54.35 ± 23.06% of the workers were smokers while, the number was 21.74 ± 11.88% for the control group (P < 0.05).
8-OHdG levels
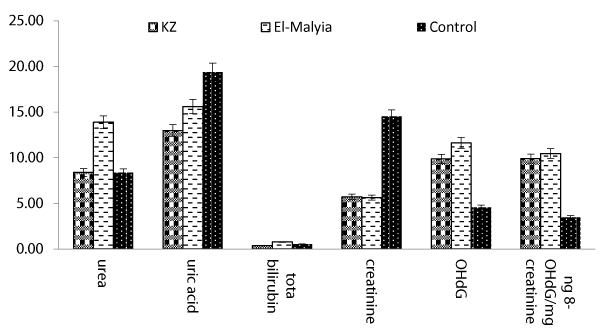
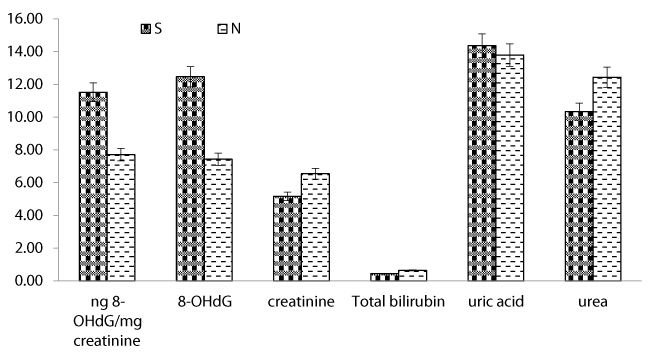
The mean values of measured variables e.g. 8-OHdG, urea, uric acid, total bilirubin, and creatinine of studied groups are illustrated in figure 1. Exposed workers of KZ Co. revealed the range from 2.55 to 25.98 ng/ml for 8-OHdG with a mean value (10.30 ± 1.72 ng/ml). However, it reached the highest peak value 10.29 ± 1.60 ng/mg creatinine. However, the values for El-Malyia workers exhibited a range of (2.86-36.43 ng/ml) with a mean value (12.47 ± 2.61 ng/ml) compared with reference group which had not exceed 4.58 ± 1.03 ng/ml (P < 0.01).
Biochemical variables
Other measured variables in the two exposed worker groups revealed a significant difference in comparison with control group. Urea levels ranged from 1.99 to 14.91 mg/dL with a mean value of 8.39 ± 0.84 mg/dl in urine samples collected from workers of KZ Co. Whereas the group of El-Malyia Co., the values ranged from 4.41 to 30.49 mg/dl with a mean value of 13.89 ± 1.63 mg/dl compared with the control group which exhibited a mean value; 8.37 ± 1.70 mg/dL (P < 0.05). Similarly, uric acid levels in samples of KZ Co. were lower than those of El-Malyia Co., where the 1st group exhibited a range from 6.78 to 23.47 mg/dL with a mean value of 12.98 ± 1.01 mg/dL, but the 2nd group exhibited a range of (10.04-21.46 mg/dL) with a mean value; 15.61 ± 0.65 mg/dl. However, the control group exhibited a mean value; 19.39 ± 1.48 mg/dL (P<0.01). Regarding total bilirubin levels, samples of KZ group exhibited a range from 0.11 to 2.44 mg/dL with a mean value; 0.36 ± 0.12 mg/dL, while the 2nd group (El-Malyia Co.) exhibited a range of (0.14-3.62 mg/dL) with a mean value; 0.78 ± 0.23 mg/dL. The control group exhibited a mean value of (0.54 ± 0.17 mg/dL). Creatinine showed varied levels in the exposed groups compared with the control. In case of KZ group, the levels revealed a range from 1.4 to 15.2 mg/kg/24 hr with a mean value; 5.72 ± 0.98 mg/kg/24 hr, but El-Malyia group exhibited a range from 1.2 to 12.8 mg/kg/24hr with a mean value; 5.62 ± 0.81 mg/kg/24hr. However, the control group gave a mean value; 14.52 ± 0.39 mg/kg/24hr.
Biochemical measurements in correlation with participant's characteristics
The correlation between the measured biochemical variables and participant's characteristics were evaluated by using a simple linear model.
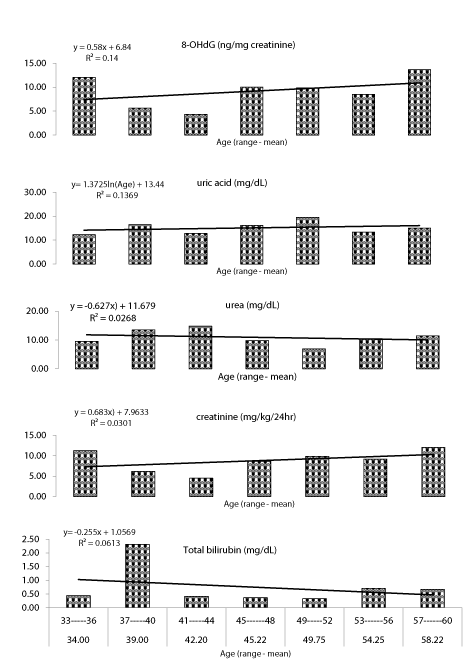
Age: The correlation concern measured parameters with exposed worker's ages are illustrated in figure 2. A significant correlation existed between 8-OHdG levels and age (y = 0.58x + 6.84, r2 = 0.14). The group aged from 57-60 yr revealed the highest level followed by (33-36) and (45-48 yr) group. Uric acid levels exhibited a significant correlation with aged groups (y = 1.37 + 13.44, r2 = 0.13). Group aged between, 49-52 yr showed the highest level followed by (45-48) and (37-40 yr). Regarding urea levels, no significant difference observed between aged groups (y = 0.627 + 11.679, r2 = 0.0268). Creatinine level showed a significant correlation with aged groups (y = 0.683 + 7.963, r2 = 0.0301). However, no significant difference obtained between group aged 33-36 yr and group of 57- 60 yr. Group ranged from 41 to 44 yr revealed the lowest creatinine level. A significant correlation existed between total bilirubin and worker ages (y =-0.255 + 1.0569, r2 = 0.063). The group aged of 37 to 40 yr old recorded the highest level than other age groups.
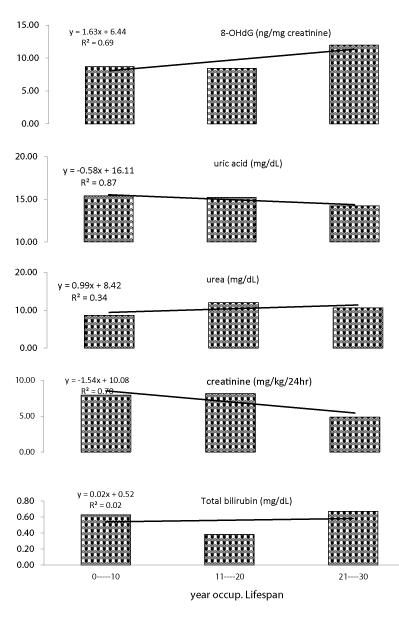
Occupational lifespan: The correlation concerning measured parameters with exposed working periods are illustrated in figure 3. Moderate correlation existed between 8-OHdG levels and lifespan categories (y = 1.63x + 6.44, r2 = 0.69). Moreover, 3rd group (21-30 yr) exhibited the highest level of 8-OHdG (11.97 ng/mg creatinine), but no significant difference obtained between other groups 1st (0-10 yr) and 2nd (11-20 yr). Concerning uric acid level, a moderate correlation was obtained between biochemical levels and three worker's categories (y = 0.58x + 16.11, r2 = 0.87). Moreover, no significant difference was observed between the 1st and 2nd group. Urea level existed slight correlation with worker categories (y = 0.99x + 8.42, r2 = 0.34). In case of creatinine, a significant correlation was recorded for worker's categories (y = 1.54x + 10.08, r2 = 0.70). The second group (11-20 yr) exhibited the highest value; 8.16 mg/kg/24hr followed by the 1st and 3rd group, respectively. Total bilirubin levels correlated significantly between worker's categories (y = 0.02x + 0.52, r2 = 0.02). No significant difference was found between the 1st and 3rd group.
Exposure period: The correlation between measured biochemical parameters and work exposure period is presented in table 2. Mean values of biochemical variables were significantly different than non-exposed group (control) (P < 0.05). The mean values of 8-OHdG levels were in the following order: 9.05 ± 2.07; 10.08 ± 2.31 and 10.67 ± 2.45 ng/mg creatinine, for exposure periods 1, 5 and 6 days/week, respectively. On the other hand, the lowest value was recorded after 4 days/week (2.99 ± 0.69 ng/mg creatinine). Regarding uric acid levels, the highest mean value was after 1 day/week (19.12 ± 1.39 mg/dL) followed by (16.65 ± 1.21 mg/dL) after 4 days/week. However, the lowest one was recorded after 6 days/week, 13.82 ± 1.01 mg/dL compared with control group (19.39 ± 1.41 mg/dl). Concerning urea levels, the mean values exhibited the following order: 4>5>6>1 day/week to be 12.52 ± 1.24; 11.22 ± 1.11; 11.15 ± 1.10 and 7.15 ± 0.71 mg/dL, respectively, compared with control mean value; 8.37 ± 0.83 mg/dl. Mean values of total bilirubin did not exceed 1 mg/dL. On the other hand, urinary creatinine levels of exposed groups exhibited mean values lower than the control group; 14.52 ± 3.62 mg/kg/24hr.
Other jobs: The correlation between biochemical variables and additional activities regarding exposed workers are presented in table 3. A significant difference was observed between worker's categories with biochemical variables (P < 0.05). The highest mean value of 8-OHdG was recorded for the farmers group (14.99 ± 3.68 ng/mg creatinine), while the commercial group exhibited the lowest value; 4.49 ± 1.10 ng/mg creatinine compared with the control group which did not exceed 3.48 ± 0.86 ng/mg creatinine. A slight difference was recorded between worker's categories along uric acid levels as follows: industrial only> sprayer> commercial> farmer with mean values; 14.81 ± 1.09, 15.18 ± 1.12, 13.25 ± 0.98 and 13.31 ± 0.98 mg/dL, respectively. The control group exhibited the highest mean value; 19.39 ± 1.43 mg/dL. The industrial group exhibited the highest mean value; 11.61 ± 0.80 mg/dL for urea level, while farmer group exhibited the lowest value; 8.18 ± 0.56 mg/dL. No significant difference obtained between bilirubin mean values which did not exceed 1 mg/dL. Finally, creatinine mean values accounted for a range from 2.40 ± 0.65 to 10.20 ± 2.74 mg/kg/24hr, where the highest mean value was recorded for the commercial group, and the lowest value was recorded for farmer group. Reference group (control) exhibited the mean value; 14.52 ± 3.90 mg/kg/24hr.
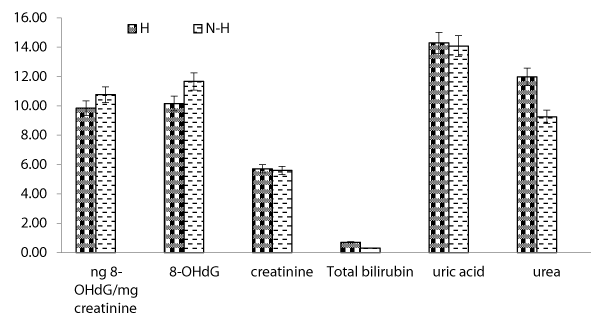
Prevalence of hepatitis C: The correlation between worker's hepatitis status and biochemical variables are illustrated in figure 4. 8-OHdG levels showed moderate differences between hepatitis worker's group and non-hepatitis subjects with mean values; 9.84 ± 2.80 and 10.76 ± 3.07 ng/mg creatinine (P < 0.05), respectively. Similarly, uric acid levels exhibited the same pattern with mean values; 14.30 ± 1.56 and 14.07 ± 1.53 mg/dL, while urea levels of hepatitis group exhibited mean value; 11.97 ± 1.32 mg/dl higher than those found in non-hepatitis (9.25 ± 1.02 mg/dL). Total bilirubin levels in either group's recorded mean values did not exceed 1 mg/dl. Also, no significant difference obtained in either group, where creatinine levels showed mean values; 5.71 ± 1.96 or 5.60 ± 1.92 mg/kg/24hr for hepatitis and non-hepatitis subjects, respectively.
Smoking habits: The correlation arising between smoking habits and biochemical variables are illustrated in figure 5. Smoking workers exhibited 8-OHdG levels highest than those of non-smokers and control group with mean values; 11.51 ± 3.53, 7.71 ± 2.36 and 3.48 ± 1.07 ng/mg creatinine, respectively. Concerning uric acid levels, moderate differences were obtained between studied subjects (P < 0.05) with mean values; 14.36 ± 1.61, 13.78 ± 1.55 and 19.39 ± 2.18 mg/dL for smokers, non-smokers and control group, respectively. However, urea levels exhibited the highest level; 12.43 ± 1.40 mg/dL in non-smokers followed by smokers (10.33 ± 1.17 mg/dl) and control group (8.37 ± 0.95 mg/dL) (P < 0.05), respectively. Bilirubin levels showed mean values no more than 1 mg/dl. In case of creatinine, the mean value of control group was 14.52 ± 4.84 mg/kg/24hr followed by non-smokers (6.55 ± 2.18 mg/kg/24hr) and smokers (5.17 ± 1.72 mg/kg/24hr) (P < 0.05), respectively.
The correlation between subject's knowledge and awareness of chemical hazards and biochemical variables are presented in table 4. The non-aware group exhibited a mean value of 8-OHdG level; 10.17 ± 4.98 ng/mg creatinine compared with control subjects (3.48 ± 1.71 ng/mg creatinine). A significant difference was obtained for uric acid levels with mean values 14.22 ± 2.19 and 19.39 ± 2.98 mg/dL for non-aware workers and control group (P < 0.05). In contrast, the mean value of urea level exhibited the highest level; 10.99 ± 1.49 mg/dL for non-aware group comparing with a control group (8.37 ± 1.13 mg/dL). No significant difference was recorded for total bilirubin levels (P < 0.05). Creatinine level recorded the lower value; 5.67 ± 2.49 mg/kg/24hr for non-aware group than those of control group (14.52 ± 6.36 mg/kg/24hr) (P < 0.05).
Discussion
The present results show statistically significant differences in the urinary biomarkers and 8-OHdG levels in exposed workers compared with control group. These findings indicate that biochemical alteration and DNA damage are associated with chemical exposure. Regarding 8-OHdG level, 2nd group (El-Malyia Co.) revealed values higher than those of KZ Co. group. The exposed workers exhibited mean values 2.45-2.72 folds of the control group which did not exceed 4.58 ± 1.03 ng/ml (P < 0.01). These findings demonstrate that pesticides or fertilizers and their by-products exposure can induce oxidative damage as well as reduce the levels of cellular antioxidant and antioxidant enzyme activity [28]. Therefore, the studied subjects of the two factories may carry an enhanced body burden of reactive genotoxic agents leading to increased frequencies of cytogenetic alterations. Moreover, the obtained data indicate that trace elements, oxides and heavy metals in fertilizer's products imposed DNA oxidation more than those induced by pesticides exposure. These findings may be explained as mentioned by [29], where Reactive Nitrogen Species (RNS) e.g. peroxynitrite, nitrous acid, and hypochlorite are able to impose DNA adduct more than Reactive Oxygen Species (ROS) which are generated from pesticides. Moreover, the work in the plant of fertilizers may induce a higher blood and urinary cadmium levels of 7.8 and 10 times, respectively, than those in a control population [5]. In fact, the presence of metals in biological systems in an uncomplexed form can significantly increase the level of oxidative stress, where the major portion of long-term effects is leading to DNA damage [30]. So, occupational and environmental exposure to metals is closely associated with an increased risk of various cancers. The occupational exposure to nitrous and sulpho-oxide gases is associated with increased oxidative DNA damage, and the level of exposure plays a critical role in this regard [31]. On the other hand, some studies demonstrated that the exposure to pesticides such as dithiocarbamates, atrazine and malathion for extended periods of time can cause DNA damage e.g. chromosomal breaks, a centric fragment, and micronucleus frequency [32]. In addition, workers exposure to polycyclic aromatic hydrocarbons, chromium (VI), residual oil fly ash and benzene, respectively, displayed increased urinary 8-OHdG concentrations [26,33,34].
Long-term of occupational exposure to pesticides and fertilizers is capable of generating ROS or RNS attack DNA, generating intermediates, which can react with DNA and form adducts such as 8-OHdG [35]. Nevertheless, several reports investigated the potential role of 8-OHdG in disease induction. The weight of evidence strongly suggests a link between such damage and the pathogenesis of the disease. It includes cancer, Parkinson's disease, Alzheimer's disease, atherosclerosis, heart failure and others. Moreover, oxidative stress is likely to be involved in age-related development of cancer. The reactive species produced in oxidative stress can cause direct damage to DNA and are considered mutagenic. It may also suppress the apoptosis [36]. All these findings indicate that 8-OHdG is a sensitive indicator for chemical occupational exposure assessment.
The results of the present study reveal that occupational exposure to chemicals increase urea levels of the exposed groups than the control group, but no significant differences was obtained in case of uric acid and total bilirubin levels. Most previous studies focused on biochemical alterations in blood samples of workers exposed to agrochemicals, but there isn't any investigation regarding urine analysis. On the other hand, excessive exposure for studied participants to chemicals imposes significant decrease in urinary creatinine compared with control group. The selected parameters may provide information concerning the impact of occupational exposure on vital organs, e.g. kidney. In fact, excessive exposure to pesticides, heavy metals, gases, dust, and others may cause adverse changes in the hepatic, and renal biochemical markers. Salih (1995) [36] evaluated the hepatic and nephrotoxic effects of dimethoate and diazinon on rats, where uric acid, creatinine, Alanine Transaminase (ALT) and Aspartate Transaminase (AST) increased compared with control animals. It was found that pesticides elevate blood uric acid level by altering kidney function. On the other hand, Patil et al. [37] stated that occupationally exposed pesticide sprayers of grape gardens had an increase in serum bilirubin, creatinine, blood glucose and urea. These findings were associated with no proper protection measures in the fields, and the people were non-aware of these toxicants. Similarly, Ejigu and Mekonnen [38] assessed the health status attitude and level of awareness of safe pesticide handling practices of farm workers engaged in the application of pesticides on agricultural farms. The liver function tests showed elevated values. Another investigation by Khan et al [39] in Pakistan displayed that, occupational workers of traders, gardeners and owners of gardens significantly were affected by agrochemicals. They had significantly altered hemoglobin level, calcium, uric acid resulting in altering in kidney function as well as liver function.
The correlations between biochemical variables and personal characteristics display positive concept with occupational lifespan and exposure periods. On the other hand, additional activities especially farming induced high DNA damage and alterations in other biochemical variables. Prevalence of smoking habits revealed a significant increase in 8-OHdG levels than those in non-smokers. However, creatinine levels decrease in smoker group than those of non-smoker group. In fact, cigarette smoking was shown in some studies [40,41] to rise 8-OHdG levels in human cells as well as 8-OHdG excretion rates [42]. The present investigation displays the exposure to broad range of chemicals as well as smoking contents recently increase DNA damage and alter other measurements. Concerning subjects involved in the factories of pesticides and fertilizers, several studies found significant positive associations between exposure and respiratory symptoms or decreased lung function. The studies focused on the manufacture of urea [43], pentachlorophenol [44], chlorpyrifos [45], the package of liquid pesticides (pyrethroids and carbamates) [46] and various other pesticides [47].
Conclusion
From the present study, it has been concluded that worker's groups of Kafr El-Zayat district significantly employ risk of occupational exposure. The impact of pesticides and fertilizers affects DNA adducts and alters biochemical variables. 8-OHdG measurement is considered a good biomarker for assessing occupational exposure to pesticides and fertilizers.
Recommendation and limitation
Protective measures must be undertaken. The two factories were established about 50 year ago. So, renovation work must be done and the technology and the framework must be changed. Moreover, work processes associated with peak exposures should be identified. Furthermore, the workers should be educated and trained to improve their awareness about harmful effects of the chemicals.
In the present study, there are limitations of chemicals analysis in urine samples. Also, large population size and other fluid samples were not undertaken. Thus, we recommend that, more studies concern workers in the manufacture of pesticides and fertilizers in Egypt should be done to establish health promotion, personal survival and good exposure assessment or biomonitoring programing.
- Maroni M, Fanetti AC, Metruccio F. Risk assessment and management of occupational exposure to pesticides in agriculture. Med Lav. 2006; 97: 430-437. https://bit.ly/2pf0MzH
- Baldi I, Lebailly P, Rondeau V, Bouchart V, Blanc Lapierre A, Bouvier G, et al. Levels and determinants of pesticide exposure in operators involved in treatment of vineyards: results of the PESTEXPO Study. J Expo Sci Environ Epidemiol. 2012; 22: 593-600. https://bit.ly/2onEtqS
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999; 107: 409-419. https://bit.ly/2nF12Hu
- Sanborn MD, Cole D, Abelsohn A, Erica Weir. Identifying and managing adverse environmental health effects: 4. Pesticides. CMAJ. 2002; 166: 1431-1436. https://bit.ly/2mKaAk2
- EPA. Environmental Protection Agency. Background report on fertilizer use, contaminant and regulations.
- Higashi T. Study on a model for future occupational health. Ind Health. 2006; 44: 541-555. https://bit.ly/2nxmsXg
- Blair A, Zham SH. Cancer among farmers. J Occup Med. 1991; 6: 335-354.
- Georgellis A, Kolmodin Hedman B, Kourestas D. Can traditional epidemiology detect cancer risk caused by occupation exposure to pesticides. J Exp Clin Cancer Res. 1999; 118: 159-166. https://bit.ly/2nx4lAL
- Purdue MP, Hoppin JA, Blair A, Dosemeci M, Alavanja MC. Occupational exposure to organochlorine insecticides and cancer incidence in the agriculture healthy study. Int J Cancer. 2007; 120: 642-649. https://bit.ly/2mTNojC
- Khuder SA, Mutgi AB. Meta-analyses of multiple myeloma and farming. Am J Ind Med. 1997; 32: 510-551. https://bit.ly/2mSuZ6I
- Beck WS. Hematology. 5th edition. Cambridge Univ. Press, Cambridge, 1991; 667 pp.
- Mourad TA. Adverse impact of insecticides on the health of Palestinian farm workers in the Gaza Strip: A hematologic biomarker study. Int J Occup Environ Health. 2005; 11: 144-149. https://bit.ly/2osl9IV
- Remor AP, Totti CC, Moreira DA, Dutra GP, Heuser VD, Boeira JM, Occupational exposure of workers to pesticides: Biochemical parameters and evaluation of genotoxicity. Environ Int. 2009; 35: 273-278. https://bit.ly/2nGi8Vz
- Sharma P, Shankar S, Singh R. Variation in serum lipids & liver function markers in lindane exposed female Wister rats: Alternating effect of curcumin, vitamin C & vitamin E. Asian J Exp Biol Sci. 2010; 1: 440-444.
- Au WW, Sierra Torres CH, Cajas Salazar N, Shipp BK, Legator MS. Cytogenetic effects from exposure to mixed pesticides and the influence from genetic susceptibility. Environ. Health Perspect. 1999; 107: 501-515. http://bit.ly/2oqVsZC
- ,Zeljezic D, Garaj Vrhovac V. Chromosomal aberration and single-cell electrophoresis (comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis. 2001; 16: 359-363. http://bit.ly/2p686NJ
- Da Silva J, Moraes CR, Heuser VD, Andrade VM, Silva FR, Kvitro K, et al. Evaluation of genetic damage in a Brazilian population occupational exposed to pesticides and its correlation with polymorphisms in metabolizing genes. Mutagenesis. 2008; 35: 415-422. http://bit.ly/2oqVwbu
- Rahman MH, Bratveit M, Moen BE. Exposure to ammonia acute respiratory effects in a urea fertilizer factory. Int J Occup Environ Health. 2007; 13: 153-159. http://bit.ly/2oqmdNs
- De Souza Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, et al. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanosine DNA (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1 defective mice. Cancer Res. 2001; 61: 5378-5381. http://bit.ly/2oofSSP
- Mishra BP, Badade ZG, Kaur-Anand B, Lingidi JL, Jaiswal S. 8-Hydroxydeoxyguanosine (8-OHdG) levels in urinary samples of pesticide sprayers on exposure to organophosphorus pesticides. Int J Clin Trails. 2015; 2: 59-63. http://bit.ly/2nKjX3R
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013; 10: 3886-3907. http://bit.ly/2okRKRd
- Kisby GE, Muniz JF, Scherer J, Lasarev MR, Koshy M, Kow YW, et al. Oxidative stress and DNA damage in agricultural workers. J Agromed. 2009; 14: 206-214. http://bit.ly/2p6mUMi
- Al Qarawai, AA, Adam SE. Effects of malathion plus superphosphate or urea on Najdi Sheep. Vet Hum Toxicol. 2003; 45: 3-6. http://bit.ly/2nIUGqB
- Azmi MA, Azmi NA, Aslam M. Effect of pesticide on health and different enzymes levels in the blood of farm workers from Gadap (rural area) Karachi-Pakistan. Chemo. 2006; 64: 1739-1744. http://bit.ly/2onZX7b
- Kossomann S, Tustanowski J, Kolodzieji B. Renal dysfunction in chemical plant workers producing dust pesticides. Med Pr. 2001; 52: 253-256. http://bit.ly/2nGmlIQ
- Qarawri Kuo HW, Chang SF, Wu KY. Chromium (VI) induced oxidative damage to DNA: increase of urinary 8-hydroxydeoxyguanosine concentrations (8-OHdG) among electroplating workers. Occup. Environ Med. 2003; 60: 590-594. http://bit.ly/2nx76SE
- Gomez KA, Gomez KH. Statistical Procedures for agricultural research. 2nd Edition. 1984; New York: John Willy and Sans Press.
- Norppa H. Cytogenetic biomarkers and genetic polymorphisms. Toxicol Lett. 2004; 149: 309-334. http://bit.ly/2nKsMdZ
- Halliwell B. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Free Radic Res. 29: 469-486. http://bit.ly/2orc436
- Kyung Taek R. Oxidative DNA damages by chemical exposures at work. Advan Biosci Biotechnol. 2012; 3: 957-971. http://bit.ly/2omrVjy
- Wronska Nofer T, Nofer JR, Jajte J, Dziubaltowska E, Szymczak W, Krajewski W, et al. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O). Mutat Res Fundam Molec Mech Muta. 2012; 731: 58-63. http://bit.ly/2mSYwNC
- Battershill JM. The multiple chemicals and actions model of carcinogenesis. A possible new approach to developing prevention strategies for environmental carcinogenesis. Hum Exp Toxicol. 2005; 24: 547-558. http://bit.ly/2omufqM
- Lagorio S, Tagesson C, Forastiere F, Iavarone I, Axelson O, Carere A. Exposure to benzene and urinary concentrations of 8- hydroxydeoxyguanosine, a biological marker of oxidative damage to DNA. Occup Environ Med. 1994; 51: 739-743. http://bit.ly/2p6AQ94
- Kim JY, Mukherjee S, Ngo LC, Christiani DC. Urinary 8-hydroxy-2'-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulate. Environ. Health Perspect. 2004; 112: 666-671. http://bit.ly/2oqGoea
- Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001; 48: 65-76. http://bit.ly/2mT0BJs
- Evans MD, Cooke MS. Factors contributing to the outcome of oxidative damage to nucleic acids. Bioassays. 2004; 26: 533-542. http://bit.ly/2omybb2
- Salih EMA. Toxic effect of dimethoate and diazinon on the biochemical and hematological parameters in male rabbits. Jordan J Biol Sci. 1995; 3: 77-82.
- Patil JA, Patil AJ, Sontakke AV, Govindwar SP. Occupational pesticides exposure of sprayers of gape gardens in western Maharashtra (India): effects on liver and kidney function. J Basic Clinic Physiol Pharma. 2009; 20: 335-355. http://bit.ly/2nFQvfa
- Ejigu D, Mekonnen Y. Pesticide use on agricultural fields and health problems in various activities. East Afric Med J. 2005; 82: 427-432. http://bit.ly/2omAFWU
- Khan AA, Shah MA, Rahman SU. Occupational exposure to pesticides and its effects on health status of workers in Swat, Khyber Pakhtunkhwa, Pakistan. J Biol Life Sci. 2013; 4: 43-55.
- Kiyosawa H, Suko M, Okudaira H, Murata K, Miyamoto T, Chung MH, et al. Cigarette smoking induces formation of 8-hydroxydeoxyguanosine, one of the oxidative DNA damages, in human peripheral leukocytes. Free Radic Res Commun. 1990; 11: 23-27. http://bit.ly/2nGQGHf
- Asami S, Manabe H, Miyake J, Tsurudome Y, Hirano T, Yamaguchi R, et al. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis. 1997; 18: 1763-1766. http://bit.ly/2okhvAZ
- Von Poppel G, Poulsen HE, Loft S, Verhagen V. No influence of beta carotene on oxidative DNA damage in male smokers. J Natl Cancer Inst. 1995; 87: 310-314. http://bit.ly/2p6F6W7
- Ruder AM, Yiin JH. Mortality of US pentachlorophenol production workers through 2001. Chemosphere. 2001; 83: 851-861. http://bit.ly/2pemOCw
- Burns CJ, Cartmill JB, Powers BS, Lee MK. Update of the morbidity experience of employees potentially exposed to chlorpyrifos. Occup Environ Med. 1998; 55: 65-70. http://bit.ly/2mOswtT
- Salameh P, Waked M, Baldi I. Spirometric changes following the use of pesticides. East Mediterr Health J. 2005; 11: 126-136. http://bit.ly/2ourjIz
- Zuskin E, Mustajbegovic J, Schachter EN, Kern J, Deckovic Vukres V, Trosic I, et al. Respiratory function in pesticide workers. J Occup Environ Med. 2008; 50: 1299-1305. http://bit.ly/2mPkJw1

Sign up for Article Alerts