Sublethal Toxicity of Thiamethoxam Insecticide in Albino Mice: Biochemical, Oxidative Damage and Histopathological Evaluations
Gamal Elsayed Abouelghar1*, Rania Ibrahim Yassien2, Zeinab Abd-Elghany El-Bermawy1, Hager Ali Ammar1 and Yassmin Abd-Elaziz Shalaby1
1Department of Pesticides, Faculty of Agriculture, Menoufia University, Shebin Elkom, MNF 32511, Egypt
2Histology and Cell Biology Department, Faculty of Medicine, Menoufia University, Shebin Elkom, MNF 32511, Egypt
*Address for Correspondence: Gamal Elsayed Abouelghar, Department of Pesticides, Faculty of Agriculture, Menoufia University, Shebin Elkom 32511, Egypt, Tel: +20-100-785-0015; ORCID ID: https://orcid.org/0000-0003-0256-3563; E-mail: gamal.abouelghar@agr.menofia.edu.eg
Submitted: 18 May 2020; Approved: 29 June 2020; Published: 30 June 2020
Citation this article: Abouelghar GE, Yassien RI, Abd-Elghany El-Bermawy Z, Ammar HA, Abd-Elaziz Shalaby Y. Sublethal Toxicity of Thiamethoxam Insecticide in Albino Mice: Biochemical, Oxidative Damage and Histopathological Evaluations. Adv J Toxicol Curr Res. 2020;4(1): 017-028. doi: 10.37871/ajtcr.id33
Copyright: © 2020 Abouelghar GE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Subacute toxicity; Neonicotinoid; Haematology; Biochemical parameters; Oxidative damage; Histopathology; Ameliorative effec
Download Fulltext PDF
The Neonicotinoid insecticides are presently used in great amounts, but this can be a problem when the possible risks of occupational and environmental contamination are considered. The objective of this study was to investigate the potential adverse effects of sublethal doses of Thiamethoxam insecticide on serum biochemical, oxidative stress and histological alterations in male albino mice via 28-day repeated-dose oral toxicity study. The possible ameliorative effect of selenium plus vitamin E against the harmful effects of Thiamethoxam was also investigated. Mice in Thiamethoxam-treated groups received three sublethal doses (6, 12, and 30 mg/kg b.w./day). Animals in another group were orally co-administered selenium + vitamin E with the higher dose of insecticide. The results showed that Thiamethoxam significantly (p < 0.05) increased cholesterol levels and liver enzyme activities, in dose-dependent manner, compared to those of the control group. Levels of creatinine were not significantly changed, whereas uric acid increased at high doses. The oxidative stress parameters were significantly increased in association with decrease in total antioxidants level. The histological analysis revealed that the higher dose induced various alterations in tissues of vital organs, i.e. liver, kidney, lung and testes. Interestingly, the ameliorative effect of selenium + vitamin E in restoring the oxidative stress parameters was reflected by reducing severity of histopathological lesions. In conclusion, it appears that the sublethal dose < 6.0 mg/kg b.w./day, in repeated dose 28-day oral toxicity study, in male albino mice may be considered as No-Observed-Adverse-Effect-Level (NOAEL) of Thiamethoxam. Additionally, the antioxidant selenium, in mixture with vitamin E, showed an ameliorative effect against Thiamethoxam-induced toxicity.
Introduction
Neonicotinoids represent a novel class of synthetic insecticides that have quickly become the most broad-spectrum class of insecticides used recently in the world because their exclusive chemical, physical and biological properties presume moderate toxicity to mammals, low application of field rates, excellent uptake and translocation in plants [1]. Within the last two decades of their commercialization, neonicotinoids have rapidly replaced the conventional insecticides, i.e. carbamate and organophosphate pesticides as a solution to human toxicity concerns and development of insect resistance from these older pesticide classes. It is known that the toxicity of neonicotinoids is attributed to its action on nicotinic Acetylcholine Receptors (nAChRs), particularly the α4β2 subtype, which are members of a ligand-gated ion channels-superfamily causing quick excitatory-cholinergic neurotransmission [2,3]. Neonicotinoids act as agonists of the postsynaptic insect-nAChRs that show significantly higher affinity binding in insect-nAChRs than in mammalian-nAChRs [4]. Therefore, the neonicotinoids are presently used in great amounts, but this can be a problem when the possible risks of occupational and environmental pollution are figured. Though the data about the mechanism of neonicotinoids on insect-nAChRs are just clear, there are a scarce works that establish possible In vivo effects of this class of insecticides on the mammalian biological system [5]. For example, Rodrigues, et al. [6] revealed that TMX might induce adverse effects at the cholinergic system of rats, producing some behavioural and biochemical alterations.
Thiamethoxam (TMX), (EZ)-3-(2-chloro-1,3-thiazol-5-ylmethyl)-5-methyl-1,3,5 oxadiazinan-4-ylidene (nitro) amine (IUPAC), TMX is the first available commercial compound (ACTARA®) that belongs to second-generation of neonicotinoids from the thianicotinyl subclass. TMX has excellent insecticidal activity properties and commonly used against a wide range of economically important pests [7,8].
A majority of subacute and/or subchronic toxicity studies focused on another neonicotinoid, imidacloprid [9-11], while a little work has been done to study the TMX-induced toxicity in mammals. However, in recent years, more concern about the possible adverse effects, involving behavioral, biochemical and histopathological alterations, which occurred due to direct or indirect exposure to sublethal-doses of TMX have been investigated by scientists. An oral administration of TMX in mice was found to reduce the level of plasma cholesterol, an earlier biomarker of liver dysfunction, which probably associated to the later histopathological changes, and eventually increased cell proliferation, cell death and tumor formation [12,13]. More recently, oral administration of sublethal doses of TMX in albino mice via subchronic toxicity study, for 6 weeks, altered some biochemical markers which correlated with histopathological symptoms in the kidney, liver, and brain [14]. The administration of rats to TMX induced significant elevations in most biomarkers associated with liver- and kidney-functions, i.e. AST (Aspartate Aminotransferase) and ALT (Alanine Amino-Transferase), ALP (Alkaline Phosphatase), glucose, urea, creatinine, and Lactate Dehydrogenase (LDH) [14-16]. Likewise, treatment of albino rats with neonicotinoid, imidacloprid, increased hepatic Lipid Peroxidation (LPO) which went together with a remarked reduction in the levels of enzymatic antioxidants [17].
Oxidative stress has been an important issue in mammalian toxicity [18] in which neonicotinoid insecticides may be involved directly in this process [19-21]. Oxidative stress as defined by Almroth, et al. [22] refers to an imbalance in pro-oxidants and antioxidants within an organism which probably lead to damage. Exposure to pesticides lead to production of Reactive Oxygen Species (ROS) which include free radicals with unpaired-electrons, e,g, Hydroxyl Radical (• OH), Superoxide Anion (O2•−), as well as non-radical molecules, Singlet Oxygen (1 O2) and Hydrogen Peroxide (H2 O2). The existence of such unpaired electrons makes the molecule highly reactive [23]. Free radicals are normally neutralized by antioxidants, however, exposure to pesticides causes imbalance between the free radicals and antioxidants which lead to disturbance of cellular pathways by inhibiting various enzymes or/and receptors, and inducing oxidative DNA damage, causing oxidative stress [23]. The formation of ROS-Malondialdehyde (MDA) subsequent oxidation of polyunsaturated-fatty acids of cell membranes is considered as another marker of oxidative stress [24]. Significant increases in levels of MDA and nitric oxide in plasma of albino mice exposed orally to 1/10 LD50 of either TMX or acetamiprid were found to be corelated with decreases in activities of the enzymatic antioxidants [25]. The levels of MDA and superoxide anion radical in kidney tissues of male rats exposed to sublethal doses of TMX were significantly higher than the control indicating an oxidative damage [20]. Likewise, sublethal exposure of Wistar albino rats to imidacloprid resulted in oxidative damage which might be due to the disturbance in cellular oxidative stress as evidenced by increasing level of LPO, decreasing activities of GPX (Glutathione-Peroxidase), SOD (Superoxide Dismutase) and levels of reduced Glutathione (GSH), cytoplasmic and membrane proteins as well as histopathological changes in liver tissues [21].
It has been known that histopathological symptoms in tissues of an organism can be used as a rapid method to estimate the toxic effects of chemicals including pesticides in tissues of different organs [26]. However, studies on the histological alterations probably induced in mammals in response to exposure to TMX are still relatively rare. However, there are some researchers have focused mainly on study of histological alterations induced in response to sublethal exposure of other neonicotinoids. Among these studies, the hepatotoxicity was reported in albino rats exposed to sublethal doses of imidacloprid, including dilation and congestion of the central vein and degeneration of hepatocytes which were in rather correlated to an increase of liver-function enzymes [21,27,28]. Histological alterations were found in liver tissue of albino rats administered to TMX, including mild focal necrosis with swollen cellular nuclei and cytoplasmic lesions [14]. Also, oral exposure of albino rats to sublethal doses of TMX resulted in marked damage in kidney tissues [20].
On the other hand, more attentions have been focused on the protective/ameliorative role of natural antioxidants against chemicals-induced neurotoxicity especially whenever free radical generations are involved. It’s known that Selenium (Se) has been widely recognized as an essential trace dietary element for animals acting as an important antioxidant [29,30]. Dietary administration of selenium to albino rats prior to exposure to methyl-parathion for 8 weeks improved the histological lesion in testicular tissues induced by methyl-parathion [31]. In addition, non-enzymatic antioxidants like Vitamin E (Vit E), vitamin C and glutathione act upon the generated free-radicals. Vitamin E is considered the most significant lipid-soluble antioxidant existed in the cell antioxidant-defense system. α-Tocopherol is biologically the most active form of vitamin E and the major lipid-soluble antioxidant present in cells and blood [32], where it scavenges Lipid Peroxyl Radicals (LOO˙) and terminates the Lipid Peroxidation (LPO) chain reactions in biological systems. Several experimental studies have shown that vitamins E and C could ameliorate pesticide toxicity [33,34]. The protective role of selenium plus vitamin C was reported in albino rats administered sublethal doses of methomyl by reducing hepato-and nephrotoxicity [35]. Also, administration of selenium combined with α-tocopherol showed an ameliorative effect against malathion-induced renal and hepatic toxicity in albino rats [36].
Therefore, in our present study, the oxidative stress, haematological, biochemical and histopathological biomarkers were investigated in albino mice exposed to sublethal doses of neonicotinoid, TMX, via oral subacute toxicity study. Additionally, the ameliorative effect of selenium combined with vitamin E on TMX-induced toxicity was determined. The results of such study may help in estimating the No-Observed-Adverse-Effects-Level (NOAEL) of TMX and establishing a biomarker to neonicotinoid toxicity
Materials and Methods
Chemicals
Commercial product of thiamethoxam (TMX) (ACTARA®, 25% WG) used in this study was purchased from the local market of pesticides, Shoura Chem. Co., Giza, Egypt. The chemicals used in biochemical analyses were of commercially available BioDiagnostic grade-kits, and purchased locally from Diagnostic & Research Reagents Co., Dokki, Giza, Egypt. Selenium was obtained from local veterinary pharmaceutical company (Movartis-Agro, Egypt), at which marketed commercially as Sodium Selenite (Na2SeO3) mixed with Vitamin E (α-tocopherol acetate) (100 + 7500 mg/L, respectively).
Experimental animals
This study was conducted on adult male albino mice, 8-10 weeks of age, weighing 25-30 g purchased from the Egyptian Company for Veterinary Drugs and Vaccines (VACSERA), Helwan Farm, Egypt. The animals were maintained in a room under controlled conditions of temperature (26 ± 2°C), lighting (12-h day/night cycle), and humidity of 40-70%. During experiment, all mice had free access to water and fed daily on a commercial pelleted diet purchased from local company (Ayad Company, Sadat City, Egypt). The diet provides essential nutrients, with approximately 17.51% proteins, 5% fats, 62% carbohydrates, 3.3% calcium, and 0.48% phosphorus, to meet the requirements of breeding animals. The animals were acclimatized for two weeks in our lab, at Department of Pesticides, before using them for experimentation. The handling procedures of mice were conducted in strict conformation with the guidelines and welfare regarding animal protection approved by Local Institute Ethical Committee of our Institution (approval no. 1689/23 Aug 2018) and the European Communities Council Directive (2010/63/EU).
Acute oral toxicity
The acute oral toxicity assay was performed based on the method described by the Organization for Economic Cooperation and Development (OECD) to determine LD50 (the median lethal dose) of TMX [37]. Oral gavage needle was used for administration of tested TMX doses in mice. Initially toxicity tests were carried out using series of TMX-doses diluted in distilled water to estimate the Maximum Tolerated Dose (MTD) in pilot dose range. The pilot study so called “up-and-down” procedure was followed as described by Bruce [38]. The test doses of TMX ranged from 250.0 to 1750.0 mg Active Ingredient (AI)/kg body weight (b.w.). Twenty-five male mice, weighing 30 ± 2 g, were allocated into five groups of five animals each. Mice in each group were oral administered single dose via gavage needle. Animals in the control group were orally administered with only water. The percent mortality in each group was calculated after 24 h. Values of LD50, 95% Confidence Limits (CL) and Slope (b), were calculated by using Probit and Logit Analysis software program (Polo Plus, ver. 2.0, 2008) based on Finney analysis [39]. The acute toxicity signs of TMX induced in response to exposure to chosen doses were observed in each group for the early 2-6 h following treatment.
Sub-acute toxicity study
For repeated dose toxicity study, selected sublethal doses of TMX were administered orally for 28 consecutive days in male mice as described by OECD technique for the testing of chemicals [40]. In this experiment, sixty adult male mice, weighing 30 ± 2 g, were randomly divided into six groups of 10 individuals in each polycarbonate cage (38 × 25 × 20 cm), under controlled conditions of temperature and photoperiod with free access to food and water as described above. Each group I, served as negative control where animals were given only distilled water. The animals in TMX-treated groups: II, III and IV, were given the selected sublethal doses of TMX, i.e. 30.0, 12.0 and 6.0 mg AI/kg b.w. , corresponding to 1/20, 1/50 and 1/100 LD50, respectively, diluted in water. The test doses were calculated from the percentage of active ingredients of the commercial formulation. Mice in Group V received Selenium (Se) + Vitamin E (Vit E), at doses of 0.3 + 50.0 mg/kg b.w, respectively. Mice in Group VI were administered with Se + Vit E, at the same dosages of group V, at first, and 10 minutes later, the animals were given the higher dose of TMX (30.0 mg/kg b.w.). The animals in each group were treated once a day over a period of 28 consecutive days using oral gavage technique.
Sample collection
At the end of experiment period, on the 29th day, animals from each group were weighed and sacrificed by cervical dislocation technique. Prior to sacrifice, blood was collected directly from their heart using heparinized hypodermic syringe for hematological analysis or/and without anticoagulant to obtain serum for biochemical analysis. After sacrifice, liver, kidney, lung and testes were immediately removed, lightly blotted and weighed to record absolute and relative organ weight. The relative weight of organs (%) was calculated as grams per 100 grams body weight. Later on, these organs were used for histopathological evaluation.
Blood sample was collected in a test tube rinsed with heparin saline solution as anticoagulant, in the proportion of 50 µl for each 1.0 mL of blood and used for the complete blood count. Whole blood was used for analyses of the total Red Blood Cell (RBC), Hemoglobin content (Hb), Hematocrit (HCT), total number of White Blood Cells (WBC) or Leukocytes, Lymphocyte Percentage (LYM), and Platelet (PLT) by employing a fully automated hematology analyzer (Swelab Alfa Hematology Analyzers, Boule Diagnostics AB, Spånga Domnarvsgatan 4, Sweden).
Biochemical parameters
The serum was used for the analyses of biochemical parameters. To collect the serum, blood samples were left to clot in clean dry tubes and centrifuged at 1500 ×g for 10 min at 4° C to obtain the sera. The clear serum samples were carefully aspirated off with a fine-bore pipette to avoid extracting red cells and stored frozen at −20° C until using for biochemical analysis. All biochemical analyses were determined using commercial kits (Diagnostic & Research Reagents Co., Dokki, Giza, Egypt), and following standard procedures outlined by the producer.
Serum transaminases (also called aminotransferases), i.e. Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT), are commonly considered sensitive biomarkers of acute hepatocellular damage. The activities of both enzymes were determined colorimetrically (at 450 nm) according to the methods of Reitman and Frankel [41]. Diagnostically, the activities of these enzymes are almost measured in units/liter (IU/L).
Serum cholesterol was determined by following the technique of by Roschlau, et al. [42]. Cholesterol assay is based on cholesterol esterase hydrolysis of cholesterol esters to form free cholesterol and cholesterol dehydrogenase catalyzed conversion of cholesterol to cholest-4-ene3-one, in which NAD is reduced to NADH. The Optical Density (OD) of the formed-NADH, at 340 nm, is directly proportionate to the concentration of cholesterol in the sample. Total serum cholesterol is expressed as mg/dL.
Creatinine test is most widely biomarker used to assess kidney function. The Henry method [43] was followed to determine the level of serum creatinine. The method was based on the reaction of creatinine with alkaline picrate. The assay utilizes a kinetic absorbance method to overcome interference by colored compounds in serum. In this method, creatinine reacts with picric acid in alkaline conditions to form a color complex which absorbs at 492 nm. Level of serum creatinine is expressed as mg/dL.
Serum uric acid concentration was estimated by the method of Fossati, et al. [44]. This assay is based upon the methods of modified trinder peroxidase assay using 3,5-Dichloro-2-Hydroxy-Benzene-Sulforic Acid (DCHB). The assay was based on oxidation of uric acid into allantoin via uricase production of hydrogen peroxide which reacts with 4-amino-antipyrine and (DCHB) in the presence of peroxidase to yield a quinoneimine dye. The subchange in absorbance at 546 nm is proportional to uric acid concentration in the sample. Uric acid level is expressed as mg/dL.
Determination of oxidative damage biomarkers, i.e. lipid peroxidation and hydrogen peroxide, were performed according to the details given in BioDignostic kit’s instructions (Diagnostic & Research Reagents Co., Dokki, Egypt). Oxidative damage was estimated by measuring the degree of Lipid Peroxidation (LPO). Lipid peroxidation evidenced by formation of the Thiobarbituric Acid-Reactive-Substances (TBARS). This assay Measures Malondialdehyde (MDA), which is a split product of an endoperoxide of unsaturated fatty acids resulting from oxidation of lipid substrates [45]. The MDA reacts with a Thiobarbituric Acid (TBA) producing a pink chromogen (TBARS), which is measured at 532-535 nm. MDA concentration was expressed as nmole/mL.
Serum hydrogen peroxide concentration was estimated based on the technique described by Nourooz-Zadeh, et al. [46]. In brief, this assay utilizes the chromogenic Fe3+ -xylenol orange reaction, in which a purple complex is formed when Fe2+ is oxidized to Fe3+ by peroxides present in the sample, generating a colorimetric (585 nm) result, proportional to the level of peroxide present. The concentration of serum hydrogen peroxide was expressed as ng/mL.
Determination of total non-enzymatic Antioxidants Capacity (TAC) were performed according to the details given in BioDignostic kit’s instructions (Diagnostic & Research Reagents Co., Dokki, Giza, Egypt). The method of Sies [47] was used to measure the concentration of TAC. This procedure is based on Cu2+ ion is converted to Cu+ by both small molecules and proteins. The reduced Cu+ ion is chelated with a colorimetric probe giving a broad absorbance peak at OD 570 nm, proportional to the total antioxidant capacity. Concentration of antioxidant in sample was expressed as µmole/mL. All above biochemical analyses were conducted using UV-Spectrophotometer (Model: B01- CT-2200 Spectrophotometer, E-Chrom Tech Co., Taiwan).
Histopathological analysis
At the end of experimental period, small pieces of each liver, kidney, lung and testes were cut and kept in 10% natural formalin (pH 7.4) for fixation for 24 h at 4° C. Tissues were washed overnight in running tap water, dehydrated in ascending grades of alcohol (70% to 100%) and cleared in benzene. The fixed specimens were processed through the conventional paraffin embedding technique [48]. From the prepared paraffin blocks, 5 μm thick sections were cut, stained by Hematoxylin-Eosin (H & E), and visualized under light microscopy (Olympus) to examine the tissue architecture of the tested organs for any histopathological symptoms [48].
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed by using One-Way Analysis of Variance (ANOVA) method followed by the Least Significant Difference (LSD) test to compare means between the different treatment groups. Differences were considered statistically significant at p ≤ 0.05. All statistical analyses were made using Statistical Package for Social Sciences (SPSS) software for windows, version 17.0, Chicago, SPSS Inc.
Results
Acute toxicity and toxicity signs
The toxicity data of TMX showed that the LD50 value obtained after 24 h was 589.9 mg AI/kg body weight, with 95% Confidence Limits (CL) = 215.0 - 696.7. The regression equation was: y = 1.554+bx and the slope of regression line was 2.76. The early toxicity symptoms following acute exposure to TMX were observed within the first 30 min. The time of peak effects for TMX ranged was 3-6 h following administration by gavage. The most common symptoms were observed at the higher dose levels, 1666.6 and 1250.0 mg/kg b.w., including eye irritation, impaired pupillary function, and hair loss, convulsions, trembling, and reduced locomotor activity. However, the animals mostly recovered within 12-24 h of exposure.
Repeated dose 28-day sub-acute exposure
Body weight and internal organ weights: The data of animal body weights after 28-day repeated exposure showed that administration to the higher TMX-dose, 1/20 LD50, significantly decreased (p <0.05) the final weight of animal body compared to that of the control group, whereas the other selected doses, 1/50 and 1/100 LD50, did not cause significant changes in the mean body weights (Table 1). However, the data showed significant increases in the relative weights of liver and kidney in TMX-treated groups, as compared to these in the control group. It was obvious that the increase levels were in dose-dependent manner. Interestingly, the data showed also that oral co-administration of high dose of TMX together with Se+Vit E recovered the absolute and relative weights of liver and kidney to the normal levels in the control group (Table 1). However, the absolute and relative weights of both lung and tests were significantly decreased in groups received TMX-1/20 and 1/50 LD50 doses in comparison with those in the control. The co-administration of high dose of TMX together with Se+ Vit E slightly improved the relative weights of lung and tests compared to the control group.
Hematological indices: Results of the hematological profile in the male mice upon TMX-exposure for 28 days compared to that of the untreated control are given in table 2. The overall analysis of erythrocyte indices showed significant decreases (p <0.05) in levels of Hemoglobin (HGB), Red Blood Cells (RBC), and Hematocrit (HCT) in groups exposed to the selected doses of TMX compared with control group (p < 0.05). It seems that the decrease levels were in dose-dependent manner. Remarkably, the data showed that oral co-administration of TMX together with Se+Vit E restored the levels of HGB, RBC and HCT nearly to normal levels in the negative control (Table 2). The data also showed significant decreases (p < 0.05) in levels of Mean Cell Volume (MCV) and Mean Cell Hemoglobin (MCH) in all three groups exposed to sublethal doses of TMX, in a dose-dependent manner, compared to the control (Table 2). However, the co-administration of TMX together with Se+ Vit E recovered the levels of MCV and MCH nearly to the control levels. In addition, there was significant decrease (p < 0.05) in the levels of White Blood Cells (WBC), Lymphocyte (LYM) and Platelet (PLT) levels especially in groups exposed to TMX-1/20 and 1/50 LD50 doses, in comparison with the negative control (Table 2). The data also demonstrated that co-administration of TMX with Se+Vit E showed beneficial effect in recovering the levels of WBC, LYM and PLT nearly to these in the untreated control.
Biochemical parameters: Data of serum biochemical indices in mice exposed to TMX- sublethal doses for 4 weeks are shown in table 3. The results showed significant increases (p < 0.05) in activities of liver enzymes, aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT), in all groups exposed to TMX-doses, as compared to these in the control group. Likewise, the cholesterol concentration was highly significantly increased at 1/20 and 1/50 LD50 doses of TMX. It was obvious that the increase levels were in dose-dependent manner. The data also showed that co-administration of high dose of TMX together with Se + Vit E exhibited slight improvement in the levels of ALT, AST and cholesterol, as compared to the TMX- group alone, however these biomarkers were still significantly higher than those of the control group (Table 3). For the overall of kidney function indicators, creatinine levels in blood serum of animals exposed to selected doses of TMX were not significantly changed than that of the control group. However, uric acid levels were significantly increased at the higher doses, 1/20 and 1/50 LD50, compared to the control. The data also showed that co-administration of high dose of TMX with Se + Vit E exhibited protective effect by restoration of uric acid level to the negative control level (Table 3).
For oxidative stress parameters, the data showed clearly that the level of lipid peroxidation, as indicated by Malondialdehyde (MDA) formation, was significantly increased (p < 0.05) in mice administered high doses, 1/20 and 1/50 LD50, of TMX, compared to that of the control. Likewise, hydrogen peroxide, another important marker for the oxidative stress, was significantly increased in the groups received high doses of TMX, 1/20 and 1/50 LD50, as compared to the negative control. Interestingly, the data clearly demonstrated that administration of high dose of TMX together with Se + Vit E restored hydrogen peroxide level nearly to the control level (Table 3). On the other hand, the total antioxidant level was significantly decreased as a result of exposure to the high dose, 1/20 LD50, of TMX, whereas the other doses, 1/50 and 1/100 LD50, did not cause significant change than that of the control. However, oral co-administration of TMX together with Se + Vit E somewhat exhibited ameliorative effect by increased total antioxidant level to be nearly that of the control level (Table 3).
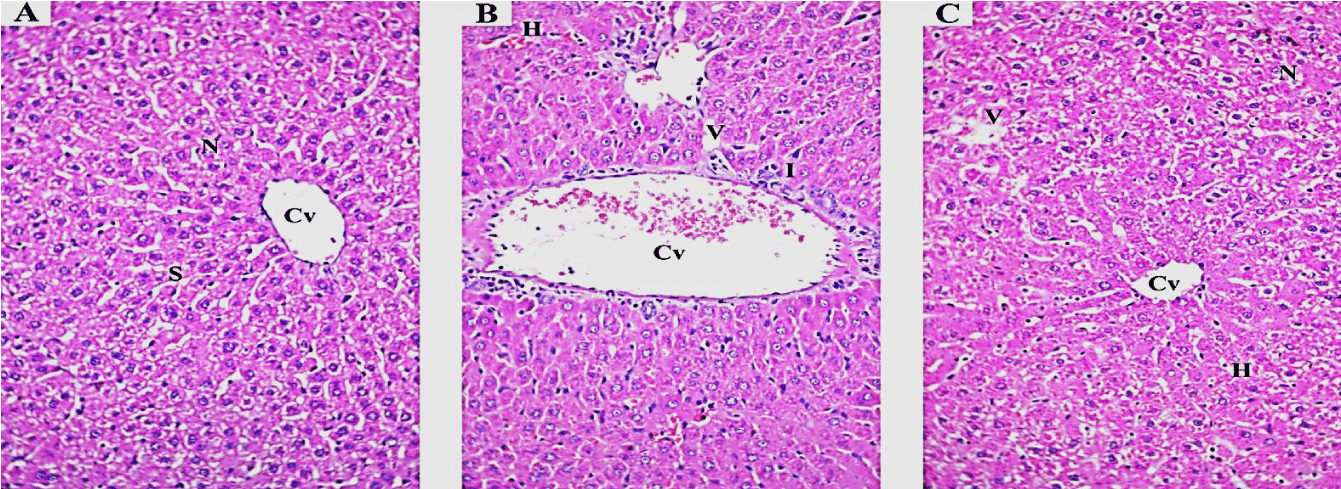
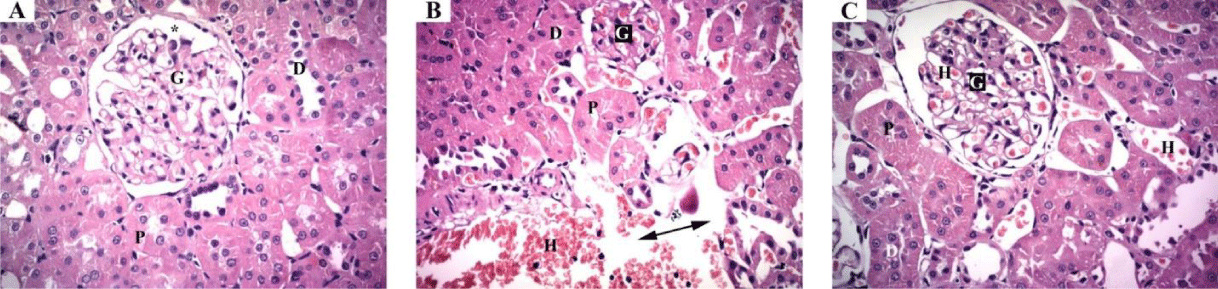
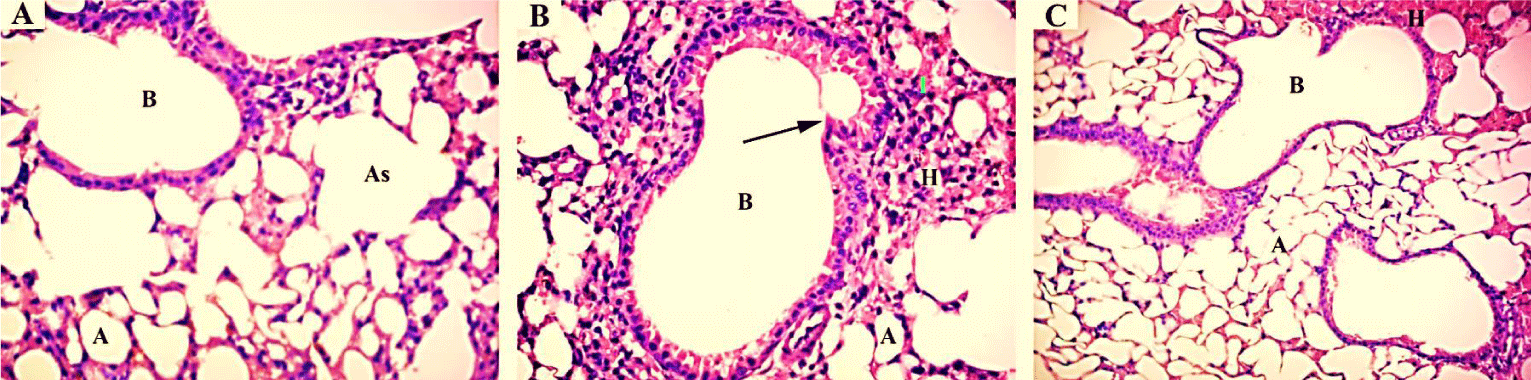
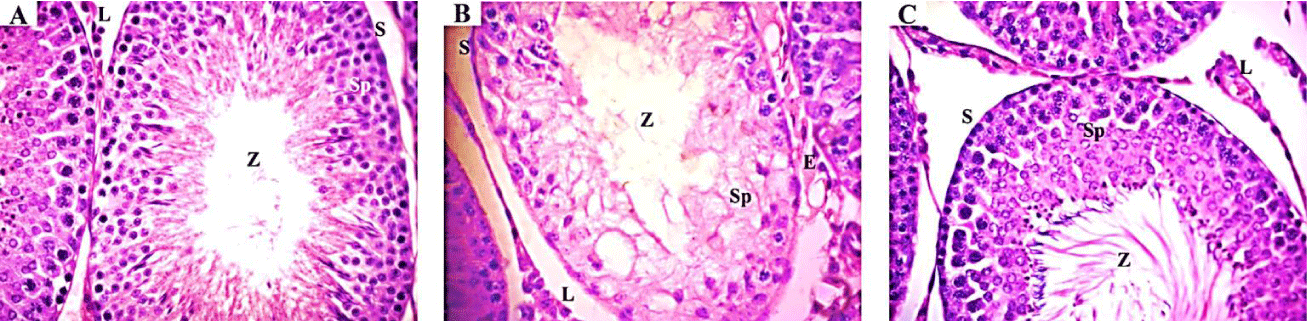
Histopathological examination: Light microscopic examination of liver from the negative control mice showed regular and compact architecture with intact portal areas, central vein, blood sinusoids and well-organized hepatocytes (Figure 1A). Hepatic tissue of mice exposed to high dose (1/20 LD50) of TMX showed dilated congested central vein with congested blood sinusoid and high infiltration of mononuclear cells. Moreover, cytoplasmic vacuolar degeneration in the hepatocytes and sinusoidal dilatation in the parenchyma were observed (Figure 1B). On the other hand, Liver tissues from mice exposed to oral co-administration of TMX + (Se + Vit E) showed nearly normal central vein surrounded with hepatocytes with vesicular nucleus, however, small vacuoles and few congested sinusoids were seen (Figure 1C). Histological examination of the kidney tissues from the control mice showed normal tissue architecture of kidney cortex, regular renal corpuscles with normal glomerulus and renal tubules (Figure 2A). The detectable lesions in the kidney of mice exposed to high dose of TMX were degeneration of the glomeruli and tubules with disorganized proximal and distal convoluted tubules. Hemorrhage and losing of tissue architecture were also observed (Figure 2B). The kidney section from the group received TMX together with (Se + Vit E) showed partial improvement in histological structure of kidney tissues with normal glomerulus, proximal and distal convoluted tubules, and less hemorrhage (Figure 2C). Histological examination of lung section of the control mice showed normal bronchiolar epithelium; the alveoli were thin-walled polyhedral chambers surrounded by single layered squamous epithelium; a thin layer of connective tissue was observed between the alveoli; an alveolus opened into alveolar sacs (Figure 3A). The detectable lesions in the lung tissues from the TMX-treated mice were dilated alveoli separated by thick end interalveolar septae with marked accumulation of RBC and congestion, as well as ruptured bronchiole with cellular infiltration were seen (Figure 3B). The lung tissues of mice received TMX in combination with Se + Vit E showed slight improvement in bronchiole and alveoli separated by thin interalveolar septae with some hemorrhage were observed (Figure 3C). Histopathological examination of testis section from the negative control showed normal well-organized seminiferous tubules, epididymis filled with sperm and Leydig cells in between the tubules (Figure 4A). Mice testes in group exposed to TMX showed lesion characterized by a disorganized seminiferous epithelium with few spermatogenic cells. Moreover, no spermatozoa in the lumen, degenerated Leydig cells and exudates in between the tubules were observed (Figure 4B). On the other hand, testes section from mice co-administered with TMX and (Se + Vit E) showed improvement and protection in testicular architecture damage with normal spermatogenesis in almost all the seminiferous tubules with some degenerated Leydig cells (Figure 4C).
Discussion
In the presented study, the 24-h LD50 value of commercial formulation of Thiamethoxam (TMX) (ACTARA®), in albino male mice, 589.9 mg AI/kg b.w., seemed to less than that reported earlier in the literature by EPA, 871.0 mg AI/kg b.w. [49] indicating that toxicity of pesticides is probably affected by the type of their formulations [50]. It is known that U.S. EPA pesticide registration is based on toxicity data of the active ingredient, not formulations as they are marketed and applied. However, some pesticide formulations are more toxic than their active ingredient (technical grade) due to the presence of active surfactants, adjuvants, or other additive components in the formulation [51,52]. The No-Observed-Effect-Level (NOAEL) reported previously for TMX in the single-dose neurotoxicity study in the 28-day dietary assay in rats was 100 ppm (equal to 8.0 mg AI kg/b.w.), based on increased plasma cholesterol concentrations [49]. Accordingly, in the present study, we selected three sublethal doses (high, middle, low), i.e. 30, 12 and 6 mg AI/kg b.w., for subacute 28-days repeated dose toxicity study. The clinical symptoms of TMX observed, in the acute toxicity study, were mostly distinguished within the first 3 h of exposure to high doses of TMX and this is reliable with the known mode of action for neonicotinoids that target Nicotinic Acetylcholine receptor (nAChR) of the postsynaptic membranes from both nerve and muscle cells and thus disrupt the transmission of the nervous influx into the central and peripheral nervous system [53]. The most observed toxicity signs of TMX reported within the first 2-3 h of administration of TMX included vomiting, nausea, agitation and multiple episodes of generalized tonic-clonic seizures [54]. Several biomarkers have been measured in the present study, including hematological indices, and serum biochemical parameters involved in liver and renal functions. Blood is considered a good biomarker for some physiological and pathological alterations of animal health [55]. The biomarker has been defined as a biological response to a chemical, or as physiological signs that reflect exposure, early cellular reaction which can provide a tactic for undertaking some toxicological issues [56,57]. Our data demonstrated that 28-day oral administration of sublethal doses of TMX in male mice led to significant decreases in levels of erythrocyte indices, mainly of RBC, HGB, and HCT, with respect to negative control. Red blood cell parameters reflect the size and hemoglobin level of the erythrocytes and help in the diagnosis of the reason of anemia. Also, leukocyte indices, involved levels of white blood cells, lymphocytes as well as platelet were significantly decreased at all selected doses of TMX. In the cellular immune response, lymphocytes are considered the most important cells of the immune response since they contain in their membrane receptors which able to distinguish certain antigenic molecules and correspond to the higher percentage of the white blood cells [58]. The hematological parameters involving HGB and Total Erythrocyte Counts (TEC) were decreased in adult cockerels exposed to TMX for 15 or 30 days [59]. Also, the total count of WBC and RBC were decreased in fish, Labeo rohita (H.), exposed to sublethal concentrations of TMX [60].
The liver has number of critical functions in the body, such as glycogen storage, plasma protein synthesis, and detoxification of xenobiotics, as well as producing bile, which is important for digestion. In the current study, significant increases in the relative weights of liver in animals exposed to selected doses of TMX were observed. Therefore, to evaluate hepatic damage, serum aminotransferase enzymes, AST and ALT, were determined. The present study demonstrated that levels of serum aminotransferases, as well as cholesterol levels in mice exposed to sublethal doses of TMX, 30 and 12 mg/kg b.w., showed significant increases with respect to the control group. Similarly, repeated-oral administration of TMX in albino rat [12,14,16] and in cockerels [59] increased the activities of liver enzymes, ALT, AST, and Alkaline Phosphatase (ALP). It is well known that the elevation of AST activity, a cytosolic enzyme of the hepatocytes, reflects the elevation of plasma membrane permeability as a result of hepatocytes lesion [61] and this is considered typically parameter of liver damage [62]. Also, the changes in the serum levels of ALT may act as a good biomarker of internal organs lesions happened especially in liver [63]. These results were also established by degeneration and necrosis of hepatocytes, which attributes an increased permeability of the cell membrane that lead to releasing of the liver aminotransaminases into the blood. These findings were almost associated with the observed damage in liver tissue architecture
The bioindicators of renal function measured in urine or/and serum are in increasing use in order to estimate the severity and nature of kidney injury. Serum creatinine, the indicator of glomerular filtration rate is still the most frequently used as biomarker of kidney function but not of the parenchymal kidney injury [64,65]. The present study showed that repeated-sublethal exposure to TMX did not cause significant difference in creatinine levels with respect to negative control; whereas, significantly increased levels of uric acid. Repeated-exposure to another neonicotinoid, acetamiprid, in albino rat for 35 days significantly increased the level of plasma uric acid, while creatinine level was not significantly changed [66]. Also, the elevation of serum urea level might be attributed to the reduction in glomerular filtration and disruption of renal tubules in the kidney [67].
Several harmful impacts on animal health are attributed to exposure to pesticides exposure, involving extreme generation of Reactive Oxygen Species (ROS). Oxygen free radicals generated as a result of pesticide exposure may cause tissue harm by activating numerous oxidative mechanisms and lipid peroxidation [68]. Since it is difficult to monitor free radicals directly In vivo, it is crucial to perform the quantification of cellular components that can react with such free radicals, e.g. proteins [69], DNA [70] and mostly lipids [71]. Increasing levels of the Lipid Peroxidation (LPO) induced by ROS has been associated with pathogenesis of several kidney and liver damages. The most commonly-used indicator of LPO is formation of Malondialdehyde (MDA) product, which is assessed by measuring the Thiobarbituric acid (TBA) [72]. The present results demonstrated clearly that 28-day repeated exposure to sublethal doses of TMX resulted in significant increase in MDA levels. Likewise, previous studies have reported that exposure of rats to TMX in subacute toxicity study significantly increased superoxide radical anion levels in liver, kidney, testis and ovary [73,74]. Additionally, the current study demonstrated that levels of hydrogen peroxide, which act as one of important oxidative stress markers, were significantly increased at all tested doses of TMX, whereas, total antioxidants levels were markedly decreased at the higher dose, 30 mg/kg b.w., of TMX. Similarly, oral administration of sublethal dose of TMX for 30 days increased the levels of MDA and Nitric Oxide (NO), whereas, decreased the activities of enzymatic antioxidants, i.e. CAT and SOD [25]. It has been known that liver damage is often associated by decline in antioxidant defenses in serum of animals exposed to pesticides. Oxidative stress also has been documented in rats exposed to sublethal doses of TMX indicating remarkable increases in levels of MDA, superoxide-anion radical and myeloperoxidase in kidney [20]. Likewise, sub-chronic administration of another neonicotinoid insecticide, acetamiprid, to guinea pigs, induced significant increases in MDA and activities of CAT and SOD, whereas reduced the level of glutathione [75].
Histopathological changes combined with oxidative stress indices in animal organs have been increasingly studied as biomarkers for toxicity of pesticides. The present study revealed that 28-day repeated oral administration of TMX, at 30 mg/kg b.w./day, showed different pathological lesions in the examined organs, viz. liver, kidney, lung and testes. The most lesion symptoms in liver tissues of mice exposed to high dose of TMX seemed to be in harmony with disturbances found in liver-function as evidenced by biochemical markers, e.g. ALT, AST and cholesterol. The histopathological alterations induced in liver and kidney of rats in response to subchronic-oral administration of TMX have been reported previously by several researchers [14,15,20]. Focal liver lesions were also detected in liver from albino mice exposed to subacute doses of imidacloprid [14].
Reports on the histopathological effects of sublethal doses of TMX on lungs and testes of mice or rats are also still scanty in the available literature. The present study showed that repeated-exposure of mice to high dose of TMX caused some histopathological changes in lungs, such as dilated bronchiole with cellular infiltration and degeneration of alveoli. These alterations might be due to the excessive formation of reactive-oxygen species caused by TMX-exposure. Likewise, oral administration of acetamiprid insecticide in Wistar albino rats induced histological alterations in lung tissues including hypertrophy of alveoli [76].
The histopathological examination of testes, in the present study, showed clearly that repeated-exposure to TMX caused damaged seminiferous tubules with no sperms in the lumen as well as detached Leydig cells in between the tubules. Similarly, an oral administration of TMX in Wistar rats caused histological alterations in tissue architecture of testes including coagulative necrosis, and depletion of the germinal epithelium [15]. However, serum level of testosterone hormone showed no significant alteration in TMX-exposed animals when compared to the control group, indicating that TMX couldn’t able to suppress testosterone production despite the detected histological lesions [77]. Another study demonstrated that administration of TMX to albino mice induced a significant decrease in the sperm viability, motility and testosterone level, with a few or no sperm in the Epididymis and incomplete mature spermatogenesis [78].
On the other hand, our present study demonstrated that oral co-administration of selenium + vitamin E with high dose of TMX exhibited additive beneficial effect to recover the levels of most hematological parameters to their normal levels. The protective effects of vitamin E, selenium and zinc against the harmful effects of cadmium- toxicity have been demonstrated in rats via improving the blood profile parameters [79]. The present data also demonstrated that co-administration of selenium + vitamin E with TMX alleviated the reduction occurred in the total antioxidant level and recovered it to the normal control value. It is known that α-tocopherol, the most potent vitamin E, is the major lipid soluble antioxidant of which protects cellular membranes and low-density lipoprotein from oxidation by inhibiting the formation of free radicals and can efficiently minimize lipid peroxidation [80,81]. Antioxidants may proliferation the immune system reactions in the organism via controlling the amount of free radicals which produced as a result of exposure to insecticides in a cell [82]. Interestingly, the current results showed that co-administration of selenium + vitamin E with TMX in mice resulted in rather positive effect on amelioration of oxidative stress by reducing the levels of MDA and hydrogen peroxide induced by exposure to high dose of TMX alone. The ameliorative action of selenium combined with vitamin C against methomyl- induced toxicity has been explained by stimulating free radical scavenger or reducing the accumulation of free radical generation with the capability to keep the normal levels of antioxidants [35,83]. Similarly, the protective role of vitamin C and vitamin E against fipronil-induced toxicity has been documented by interrupting the lipid peroxidation reaction and protecting against oxidative stress [84,85]. The protective role of vitamin E, mostly, is attributed to its ring structure possessing hydrogen giving hydroxyl group, therefore it acts as chain terminating antioxidant. The present work shows that such amelioration action of selenium combined with vitamin E in reducing oxidative stress has been reflected mostly by reducing the severity of histopathological lesions happened in liver, kidney, lung and testis tissues as a result of TMX exposure.
Conclusion
Based on the overall findings from the present study, it may be concluded that 28 days exposure of commercial formulation of TMX (ACTARA®), at 30 and 12 mg AI/kg b.w./day, induced oxidative stress as revealed by increased Malondialdehyde (MDA) and hydrogen peroxide, and disorder of total antioxidant level, serum biochemical markers as well as histological alterations. Moreover, results of this study suggest that prior administration of antioxidant vitamins E together with selenium could alleviate TMX-induced toxicity. Thus, sufficient dietary intake of such antioxidants by individuals at high risk of TMX exposure could prove beneficial in combating the adverse effects. It is suggested that the sublethal dose 6.0 mg AI/kg b.w./day, in repeated dose 28-day oral toxicity study, in male albino mice could be considered as No-Observed-Adverse-Effect-Level (NOAEL) of commercial formulation of TMX. However, further, more detailed systemic toxicity studies of TMX to better characterize its effects on immune and reproductive systems.
Funding & Acknowledgements
This research project did not receive any specific grant from funding agencies. The authors would like to thank chair of Department of Pesticides, Faculty of Agriculture, and Menoufia University for his technical assistance toward completion of this study.
Declaration of Competing Interest
The authors declare that no conflict of interest exists regarding this work. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
- Maienfisch P, Huerlimann H, Rindlisbacher A, Gsell L, Dettwiler H, Haettenschwiler J, et al. The discovery of thiamethoxam: A second-generation neonicotinoid. Pest Manag Sci. 2001; 57: 165-176. DOI: 10.1002/1526-4998(200102)57:2<165::AID-PS289>3.0.CO;2-G
- Bromilow RH, Chamberlain K. Principles governing uptake and transport of chemicals. In: Trapp S, McFarlane JC (Eds.), Plant Contamination. 1995; Lewis/CRC Press; Boca Raton, FL, USA. p. 37-64.
- Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res. 2015; 22: 35-67. DOI: 10.1007/s11356-014-3332-7.
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002; 3: 102-114. DOI: 10.1038/nrn731.
- Tomizawa M, Casida J. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Ann Rev Pharmacol Toxicol. 2005; 45: 247-268. DOI: 10.1146/annurev.pharmtox.45.120403.095930
- Tomizawa M, Lee DL, Casida JE. Neonicotinoid insecticides: molecular features conferring selectivity for insect versus mammalian nicotinic receptors. J Agri Food Chem. 2000; 48: 6016-6024. DOI: 10.1021/jf000873c
- Tomizawa M, Cowan A, Casida JE. Analgesic and toxic effects of neonicotinoid insecticides in mice. Toxicol Appl Pharmacol. 2001; 177: 77-83. DOI: 10.1006/taap.2001.9292
- Rodrigues K, Santana M, Do Nascimento J, Picanço-Diniz DL, Maués LA, Santos SN, et al. Behavioral and biochemical effects of neonicotinoid thiamethoxam on the cholinergic system in rats. Ecotoxicol Environ Safety. 2010; 73: 101-107. DOI: 10.1016/j.ecoenv.2009.04.021
- Kapoor U, Srivastava MK, Bhardwaj S, Srivastava LP. Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive it’s No Observed Effect Level (NOEL). J Toxicol Sci. 2010; 35: 577-81. DOI: 10.2131/jts.35.577
- Kapoor U, Srivastava MK, Trivedi P, Garg V, Srivastava LP. Disposition and acute toxicity of imidacloprid in female rats after single exposure. Food Chem Toxicol. 2014; 68: 190-195. DOI: 10.1016/j.fct.2014.03.019
- Green T, Toghill A, Lee R, Waechter F, Weber E, Noakes J. Thiamethoxam induced mouse liver tumors and their relevance to humans. Part 1: Mode of action studies in the mouse. Toxicol Sci. 2005a; 86: 36-47. DOI: 10.1093/toxsci/kfi124
- Green T, Toghill A, Lee R, Waechter F, Weber E, Peffer R, et al. Thiamethoxam induced mouse liver tumors and their relevance to humans. Part 2: Species differences in response. Toxicol Sci. 2005b; 86: 48-55. DOI: 10.1093/toxsci/kfi125 14.
- Arfat Y, Mahmood N, Tahir MU, Rashid M, Anjum S, Zhao F, et al. Effect of imidacloprid on hepatotoxicity and nephrotoxicity in male albino mice. Toxicol Rep. 2014; 1: 554-561. DOI: 10.1016/j.toxrep.2014.08.004.
- Khaldoun-Oularbi H, Bouzid N, Boukreta S, Makhlouf C, Derriche F, Djennas N. Thiamethoxam Actara® induced alterations in kidney liver cerebellum and hippocampus of male rats. Journal of Xenobiotics. 2017; 7: 25-30. DOI:10. 4081/xeno.2017.7149.
- El-Okle OS, Lebda MA, Tohamy HG. Thiamethoxam-induced biochemical, hormonal and histological alterations in rats. Int J Toxicol Pharmacol Res. 2016: 8: 320-325. https://bit.ly/2Vuhybi
- Shalaby SM, Farrag AR, El-Saed GS. Toxicological potential of thiamethoxam insecticide on albino rats and its residues in some organs. JASMR. 2010; 5: 165-171. https://bit.ly/2AhAp1R
- Chakroun S, Grissa I, Ezzi L, Ammar O, Neffati F, Kerkeni E, et al. Imidacloprid enhances liver damage in Wistar rats: Biochemical, oxidative damage and histological assessment. J Coast Life Med. 2017; 5: 540-546. DOI: 10.12980/jclm.5.2017j7-149.
- López O, Hernández AF, Rodrigo L, Gil F, Pena G, Serrano JL, et al. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicology Letters. 2007; 171: 146-153. DOI: 10.1016/j. toxlet.2007.05.004.
- Zhang JJ, Wang Y, Xiang HY, Li MX, Li WH, Ma KG, et al. Oxidative stress: Role in acetamiprid-induced impairment of the male mice reproductive system. Agri Sci China. 2011; 10: 786-796. DOI: 10.1016/s1671-2927(11)60063-1.
- Auwal MS, Kumar V. Effect of subchronic oral exposure of male rats to thiamethoxam, quercetin and their combination on oxidative stress parameters in kidney. BAOJ Veterinary Science. 2017; 1: 1-8. https://bit.ly/38bHNs4
- Lohiya A, Kumar V, Punia JS. Imidacloprid induced oxidative stress and histopathological changes in liver of rats. Indian J Animal Res. 2017; 51: 531-536. DOI: 10.18805/ijar.v0iOF.7805.
- Almroth CB, Albertsson E, Sturve J, Förlin L. Oxidative stress, evident in antioxidant defences and damage products, in rainbow trout caged outside a sewage treatment plant. Ecotoxicol Environ Safe. 2008; 70: 370-378. DOI: 10.1016/j.ecoenv. 2008.01.023.
- Sharma AD, Mallick AR, Ghosh AK. Free radicals and their role in different clinical conditions: An overview. Int J Pharm Sci Res. 2010; 1: 185-192. https://bit.ly/31BZYFQ
- Flohr L, Fuzinatto CF, Melegari SP, Matias WG. Effects of exposure to soluble fraction of industrial solid waste on lipid peroxidation and DNA methylation in erythrocytes of Oreochromis niloticus, as assessed by quantification of MDA and m5dC rates. Ecotoxicol Environ Safe. 2012; 76: 63-70. DOI: 10.1016/j.ecoenv. 2011.10.016.
- Keshta AT, Hataba A, Mead HM, El-Shafey NM. Oxidative stress and biochemical changes induced by thiamethoxam and acetamiprid insecticides in rats. World J Pharm Sci. 2016; 5: 44-60. DOI: 10.20959/wjpps20166-6837.
- Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J Fish Dis. 1999; 22: 25-34. DOI: 10.1046/j.1365-2761.1999.00134.x
- Toor HK, Sangha GK, Khera KS. Imidacloprid induced histological and biochemical alterations in liver of female albino rats. Pesti Biochem Physiol. 2013; 105: 1-4. DOI: 10.1016/j.pestbp.2012.10.001.
- Vohra P, Khera KS, Sangha GK. Physiological, biochemical and histological alterations induced by administration of imidacloprid in female albino rats. Pesti Biochem Physiol. 2014; 110: 50-56. DOI: 10.1016/j.pestbp.2014. 02.007.
- Battin EE, Perron NR, Brumaghim JL.The central role of metal coordination on selenium antioxidant activity. Inorganic Chemistry. 2006; 45: 499-501. DOI: 10.1021/ic051594f.
- Yousuf S, Atif F, Ahmad M, Hoda MN, Khan MB, Ishrat T, Islam F. Selenium plays a modulatory role against cerebral ischemia-induced neuronal damage in rat hippocampus. Brain Research. 2007; 1147: 218-225. DOI: 10.1016/j. brainres.2007.01.143.
- El-Gerbed MSA. Histopathological and ultrastructural effects of methyl parathion on rat testis and protection by selenium. J Appl Pharm Sci. 2013; 3: S53-S63. DOI: 10.7324/JAPS.2013.38.S9.
- Cheeseman KH, Burton GW, Ingold KU, Slater TF. Lipid peroxidation and lipid antioxidants in normal and tumor cells. Toxicol Pathol. 1984; 12: 235-239. DOI: 10.1177/019262338401200305
- Altuntas I, Delibas N, Sutcu R. The effects of organophosphate insecticide methidathion on lipid peroxidation and anti-oxidant enzymes in rat erythrocytes: Role of vitamins E and C. Hum Exp Toxicol. 2002; 21: 681-685. DOI: 10.1191/0960327102ht304oa.
- Uzunhisarcikli M, Kalender Y, Dirican K, Kalender S, Ogutcu A, Buyukkomurcu F. Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of vitamins C and E. Pesti Biochem Physiol. 2007; 87: 115-122. DOI: https://doi.org/10.1016/j.pestbp.2006.06.010
- Djeffal A, Messarah M, Boumendjel A, Kadeche L, El Feki A. Protective effects of vitamin C and selenium supplementation on methomyl-induced tissue oxidative stress in adult rats. Toxicol Ind Health. 2012; 31: 31-43. DOI: 10.1177/0748233712468020
- Al-Othman AM, Al-Numair KS, El-Desoky GE, Yusuf K, Al Othman ZA, Aboul-Soud MA, et al. Protection of α-tocopherol and selenium against acute effects of malathion on liver and kidney of rats. Afr J Pharm Pharmacol. 2011; 5: 1263-1271. DOI: 10.5897/ajpp11.226.
- OECD (Organization for Economic Cooperation and Development), harmonized integrated hazard classification system for human health and environmental effects of chemical substances. 1998; Part 2. https://bit.ly/2Bh4Zt0
- Bruce RD. An up-and-down procedure for acute toxicity testing. Fund Appl Toxicol. 1985; 5: 151-157. DOI: 10.1016/0272-0590(85)90059-4.
- Finney DJ. Probit Analysis. 1971; Cambridge, England. Cambridge University Press. https://doi.org/10.1002/jps.2600600940
- OECD (Organization for Economic Cooperation and Development), 2008. Guidelines for the testing of chemicals. Draft guidance document on acute inhalation toxicity testing. 39, 2008. https://bit.ly/2BTzB3o
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28: 56-63. DOI: 10.1093/ajcp/28.1.56.
- Roschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974; 12: 403-408. PubMed: https://pubmed.ncbi.nlm.nih.gov/4428856/
- Henry RJ. Method of creatinine. Clinical chemistry principles and techniques. 2nd ed. Harper and Row Publishers; New York. 1974.
- Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxy-benzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980; 26: 227-231. PubMed: https://pubmed.ncbi.nlm.nih.gov/7353268/
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998; 108: 101-106. DOI: 10.1385/0-89603-472-0:101.
- Nourooz Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994; 220: 403-409. DOI: 10.1006/abio.1994.1357.
- Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997; 82: 291-295. DOI: 10.1113/expphysiol.1997.sp004024
- Bancroft JD, Stevens A. Theory and practice of histological techniques. 4th ed. Churchill Livingstone; New York: 1996. https://bit.ly/3dFdU4j
- JMPR (Joint FAO/WHO Meeting on Pesticide Residues), Pesticide residues in food - 2005. Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO core assessment group. fao plant production and protection paper. 2005; 183. https://bit.ly/3gc2rLd
- Pereira JL, Antunes SC, Castro BB, Marques CR, Goncalves AM, Goncalves F, et al.Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicology. 2009; 18: 455-463. DOI: 10.1007/s10646-009-0300-y
- Bringolf RB, Cope WG, Barnhart MC, Mosher S, Lazaro PR, Shea D. Acute and chronic toxicity of pesticide formulations (Atrazine, Chlorpyrifos, and Permethrin) to Glochidia and juveniles of Lampsilis siliquoidea. Environ Toxicol Chem. 2007; 26: 2101-2107. DOI: 10.1897/06-555R.1
- Bringolf RB, Cope WG, Mosher S, Barnhart MC, Shea D. Acute and chronic toxicity of glyphosate compounds to glochidia and juveniles of Lampsilis siliquoidea (Unionidae). Environ Toxicol Chem. 2007; 26: 2094-2100. DOI: 10.1897/06-519
- Nistor N, Frasinariu OE, Ştreanga V. Acute poisoning with neonicotinoid insecticide. In: Malangu N. (Ed.), Poisoning: From specific toxic agents to novel rapid and simplified techniques for analysis. Intech Open; 2017. p: 107-123. http://dx.doi.org/10.5772/ intechopen.72004.
- Vinod KV, Srikant S, Thiruvikramaprakash G, Dutta TK. A fatal case of thiacloprid poisoning. Am J Emerg Med. 2015; 33: 310. e5-6. DOI: 10.1016/j.ajem. 2014.08.013.
- Jorum OH, Piero NM, Machocho AK. Haematological effects of dichloromethane-methanolic leaf extracts of Carissa edulis (Forssk.) Vahl in normal rat models. J Hematol Thromb Dis. 2016; 4: 1-8. DOI: 10.4172/2329-8790.1000232.
- Silbergeld EK, Davis DL. Role of biomarkers in identifying and understanding environmentally induced disease. Clin. Chem. 1994; 40: 1363-1367. PubMed: https://pubmed.ncbi.nlm.nih.gov/8013120/
- Walker CH, Hopkin SP, Sibly RM, Peakall DB. 1996. Biomarkers. In: Walker CH, Hopkin SP, Sibly RM, Peakall B Editors. Principles of Ecotoxicology. Taylor and Francis; London: 1996. pp. 175-194.
- Ruiz FS, Andersen ML, Zager A, Martins RC, Tufik S. Sleep deprivation reduces the lymphocyte count in a non-obese mouse model of type 1 diabetes mellitus. Braz J Med Biol Res. 2007; 40: 633-637. DOI: 10.1590/s0100-879x2007000500005.
- Gul ST, Khan A, Farooq M, Niaz S, Ahmad M, Khatoon A, et al. Effect of sub lethal Doses of thiamethoxam (a pesticide) on hemato-biochemical values in cockerels. Pak Vet J. 2017; 37: 135-138. https://bit.ly/2YEAG8h
- Jasmin J, Rahman MdM, Rahman MdM. Haematological Changes in Labeo rohita (H.) due to exposure of pesticides, difenoconazole and thiamethoxam. Int J Contemp Res Rev. 2018; 9: 20199-20205. DOI: 10.15520/ijcrr/2018/9/01/390
- Plaa GL, Hewitt WR. Detection and evaluation of chemically induced liver injury. In: Hayes W. Editors. Principles and Methods of Toxicology. Raven Press; New York: 1982. p. 407-445. https://bit.ly/3eKeoYl
- Klaassen CD, Eaton DL. 1991. Principles of Toxicology. In: Amdur MO, Doull J, Klaassen CD. Editors. Casarett and Doull's Toxicology: The Basic Science of Poisons, 4th ed. Pergamon Press; New York: NY: 1991. p. 12-49.
- Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals, 5th ed. Academic Press; San Diego, California: 1997. https://bit.ly/2Vn2iwL
- Martensson J, Martling C-R, Bell M. Novel biomarkers of acute kidney injury and failure: Clinical applicability. British Journal of Anaesthesia. 2012; 109: 843-850. DOI: 10.1093/bja/aes357
- Krstic D, Tomic N, Radosavljevic B, Avramovic N, Dragutinovic V, Skodric CM. Biochemical markers of renal function. Curr Med Chem. 2016; 23: 2018-2040. DOI: 10.2174/0929867323666160115130241
- Noaishi M, Abd-alhafez HH. Hepatotoxicity and nephrotoxicity evaluation after repeated dose of acetamiprid in albino rats. Egyp J Chem Environ Health. 2016; 2: 439-452. https://bit.ly/2BMXJoA
- Walmsley RN, White GH. A guide to diagnostic clinical chemistry. 1994; Blackwell Scientific Publication, London, UK. https://bit.ly/31n6YGN
- Kanbur M, Atalay O, Ica A, Eraslan G, Cam Y. The curative and antioxidative efficiency of doramectin and doramectin+vitamin AD3E treatment on Psoroptes cuniculi infestation in rabbits. Res Vet Sci. 2008; 85: 291-293. DOI: 10.1016/j.rvsc. 2007.10.005.
- Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. The American Journal of Clinical Nutrition. 1993; 57: 715-725. DOI: 10.1093/ajcn/57.5.715S.
- Seto H, Akiyama K, Okuda T, Hashimoto T, Takesue T, Ikemura T. Structure of a new modified nucleoside formed by guanosine-malondialdehyde reaction. Chemistry Letters. 1981; 52: 707-708. DOI: 10.1246/cl.1981.707.
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods in Enzymology. 1990; 186: 407-421. DOI: 10.1016/0076-6879(90)86134-h.
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde, and related aldehydes. Free Radical Biol Med. 1991; 11: 81-128. DOI: 0.1016/0891-5849(91)90192-6.
- Nigam A. Studies on subacute toxicity of thiamethoxam in male rats and its amelioration by quercetin with special reference to reproductive system. M V Sc. Thesis 2015; LUVAS, Hisar, India.
- Singh A. Studies on subacute toxicity of thiamethoxam with special reference to reproductive functions and its amelioration by quercetin in female rats. M.V.Sc. Thesis, LUVAS. 2016; Hisar, India. https://bit.ly/2Vq00Nu
- Kenfack A, Guiekep AJ, Ngoula F, Vemo BN, Bouli EP, Pamoal ET. Reproductive toxicity of acetamiprid in male Guinea pig (Cavia porcellus). J Animal Sci Vet Med. 2018: 3, 105-111. DOI: 10.31248/JASVM2018.101
- Mondal S, Ghosh RC, Karnam SS, Purohit K. Toxicopathological changes on Wistar rat after multiple exposures to acetamiprid. Veterinary World. 2014; 7: 1058-1065. DOI: 10.14202/vetworld. 2014.1058-1065.
- Breckenridge CB, Stevens JT. Crop protection chemicals: Mechanism of action and hazard profiles. In: Hayes AW (Ed.), Principles and Methods of Toxicology, 5th ed. Informa Healthcare; USA: 2008. p. 727-840.
- Hasan SL, Khalid KK, Abdulkarim JK, Jenan MK. Histopathology of male swiss albino mice reproductive system due to toxic effects of thiamethoxam. J Entomol Zool Stud. 2017; 5: 1201-1206. https://bit.ly/3dH1lFY
- Shata FY, Hassan SG, El-Nattat WS, Desouky HM, Mohamed AH, Ahmed AR. Protective effects of vitamin E, selenium and zinc supplementation on hematological and some biochemical parameters in pregnant rats exposed to cadmium. Global J Pharmacol. 2014; 8: 665-672. DOI: 10.5829/ idosi. gjp.2014.8.4.86134.
- Yavuz T, Delibas N, Yildirim B, Altuntas I, Candir O, Cora A, et al. Vascular wall damage in rats induced by methidathion and ameliorating effect of vitamins E and C. Arch Toxicol. 2004; 78, 655-659. DOI: 10.1007/s00204-004-0593-9
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007; 43: 4-15. DOI: 10.1016/j.freeradbiomed.2007. 03.024.
- Niki E. α-Α-tocopherol. In: Cadenas E, Packer L (Eds.), Handbook of Antioxidants. Dekker Inc; New York, USA: 1996. p. 3-25.
- Eraslan G, Saygi S, Essiz D, Aksoy A, Gul H, Macit E. Evaluation of aspect of some oxidative stress parameters using vitamin E, proanthocyanidin and N-acetylcysteine against exposure to cyfluthrin in mice. Biochem Physiol. 2007; 88: 43-49. DOI: https://doi.org/10.1016/j.pestbp.2006.08.010
- Badgujar PC, Chandratre GA, Pawar NN, Telang AG, Kurade NP. Fipronil induced oxidative stress involves alterations in SOD1 and catalase gene expression in male mice liver: protection by vitamins E and C. Environ Toxicol. 2016; 31: 1147-1158. DOI: 10.1002/ tox.22125.
- Abouelghar GE, El-Bermawy ZA, Salman HMS. Oxidative stress, hematological and biochemical alterations induced by sub-acute exposure to fipronil (COACH®) in albino mice and ameliorative effect of selenium plus vitamin E. Environ Sci Pollut Res. 2020; 27: 7886-7900. DOI: 10.1007/s11356-019-06579-9.

Sign up for Article Alerts