Hormonal Therapy for Prostate Cancer: Modalities and Evaluation of Results
Babacar Sine*, Ndeye A. Bagayogo, Yaya Sow, Alioune Sarr, Amath Thiam, Cirille Ze Ondo, Boubacar Fall, Samba T. Faye, Abdou Razack Hamidou Zakou, Modou Ndiaye, Ndiaga S. Ndour, Babacar Diao, Alain K. Ndoye, Mamadou Ba
Urology and Andrology department of CHU Aristide Le Dantec de Dakar-Senegal
*Address for Correspondence: Babacar Sine, Urology and Andrology department of CHU Aristide Le Dantec de Dakar-Senegal, Tel: +002-217-744-37657, E-mail: papesine@yahoo.fr
Submitted: 29 January 2018; Approved: 09 March 2018; Published: 10 March 2018
Citation this article: Sergio M, Bertozzi MA. A Brief Historical Survey of the “Contagium Vivum) (From Marcus Terentius Varro’s (116-54 B.C.) “Animalia Minuta” And Titus Lucretius Carus’ (C.94-C.54 B.C.) “Semina” To Louis Pasteur’s (1822-1895) and Robert Koch’s (1843-1910) “Germs” and “Bacteria”. Am J Urol Res. 2018;3(1): 001-009.
Copyright: © 2018 Sine B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Objective: To report the hormone therapy methods proposed to our patients, to evaluate results using the total PSA, overall survival and progression - free survival and reporting complications of hormonal treatment.
Patients and methods: This was a retrospective study of 70 patients followed from 1 June 1st 2010 to October 31st 2012 for prostate cancer with hormone therapy. Hormone therapy was medical, using LHRH analogues and antiandrogen; or surgical, using bilateral testicular pulpectomy or orchiectomy.
Results: The median age of patients was 70.5 years (48-89). Sixty - seven point one patients (67.1%) had an impair general condition (ECOG 3 and 4). The median initial total PSA ratio was 303.5 ng/ ml (0.6 to 12.990). Surgical castration was performed in 55 patients (78.6%). Sixteen patients had a complete androgen blockade immediately. The median PSA nadir was 15 ng/ ml (0.029 to 503). The median time to reach the nadir (DAN) was 4 months (3-22). Also overall survival at 6 and 12 months was 46.2% and 23.1% respectively. Progression-free survival at 6 and 12 months was 54.2% and 50% respectively. Hot flashes and anemia were the most reported complications
Conclusion: Mortality from prostate cancer remains high despite improvements in diagnostics, therapeutics and treatment monitoring.
Introduction
Prostate cancer is the leading cancer in older men and the second leading cause of death (after lung cancer). It is the fourth leading cause of death due to cancer in the general population [1]. In Africa, prostate cancer is diagnosed primarily at locally advanced or metastatic stage [2]. Hormone therapy is the standard treatment indicated in these advanced stages. However, the drugs used are not accessible (high cost) for the majority of patients with low socio-economic levels. Surgical castration is still the most used therapeutic method [3-5]. The goals of the study were to report the different hormone therapy regimens offered to our patients and the procedures of establishment, assess the oncological results using as judgment criteria: total PSA, overall survival and progression free survival and report the complications related to hormonal therapy.
Patients and Methods
This was a retrospective study carried out from September 1st, 2010 to October 31st, 2012. It collected all patients who had hormone therapy for prostate cancer confirmed histologically. The parameters studied were: patient’s age at diagnosis, patient’s general condition (ECOG performance status), Gleason score, tumor status (TNM 2009), type of treatment and treatment complications.
Hormone therapy was:
• Either medical, using analogs of LHRH (Goserelin, Triptorelin), non-steroidal antiandrogens (bicalutamide), steroidal anti-androgen (cyproterone acetate)
• Or surgically, using bilateral testicular pulpectomy or orchiectomy.
Body hygiene measure (stop all smoking, regular physical activities) were recommended for all patients. Calcium and vitamin D3 prescription was made for all osteopenic or osteoporotic patients on bone densitometry. Patients were reviewed at three, six and 12 months with a PSA control, liver function tests and Complete Blood Count (CBC). Thus, the appreciation criteria of treatment outcomes were total PSA level, overall survival, progression free survival. The original date was the date of the initiation of hormonal therapy. The data were analyzed and exploited by excel 2010 and SPSS 19.0. Survival was measured according to Kaplan Meier method. The comparative analysis of the parameters was made with a significance level of the tests (student and Khi2) set at 5%. The study was approved by the local ethics committee.
Results
The median age of patients was 70.5 years (range 48 - 89 years). Sixty - two point six percent (62.6%) of patients were over 70 years (Table 1). The general condition of patients was assessed using the ECOG performance status. Sixty-seven point one patients (67.1%) had an impaired general condition (ECOG 3 and 4). Sixty-seven point one patients (67.1%) had PSA levels greater than 100 ng/ ml. The median of initial PSA was 303.5 ng/ ml (0.8 to 12.990 ng/ ml). The Osteodensitometric status was known in 17 patients. They had a high risk of bone fractures in 70.6%. Moreover 71.4% of osteoporotic patients had metastatic cancer and the average age of these patients was 66.4 years. Surgical castration was performed in 55 patients (78.6%). This surgical castration was carried out by bilateral testicular pulpectomy in almost all cases. Only one patient had a right orchiectomy for an undescended testis, associated with left testicular pulpectomy.
Sixteen (16) patients had a complete androgen blockade immediately. Regarding the chemical castration, the LHRH agonist most used was goserelin. The anti-androgen used was bicalutamide 50 mg per day. The median nadir PSA was 15 ng/ ml (0.029 to 503 ng/ ml). The median time to reach the nadir was 4 months (3 - 22 months). The average nadir was three times higher among patients who had a large tumor mass but the tumor mass did not affect the period to reach the nadir (p = 0,17).
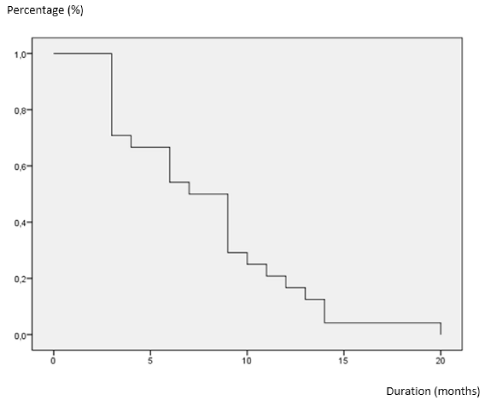
The average nadir was higher in patients with a Gleason score 6 but was reached faster. The nadir was higher among patients who had metastatic tumors but the time to reach seemed identical (P = 0,01). Also overall survival at 6 and 12 months was 46.2% and 23.1% respectively. Progression-free survival at 6 and 12 months was 54.2% and 50% respectively (Figures 1 and 2). Metastatic status seemed to adversely affect the survival of our patients. Thus the average duration of overall survival was 6.9 months for metastatic cancers and 8.6 months for locally advanced cancers. Complications were noted in 49 patients (70%). Hot flashes and anemia were the most encountered complications in our patients (Table 2).
Discussion
These high levels of PSA suggest the existence of a large tumor mass in our patient therefore to advanced disease. Niang et al. [4] have reported similar results with an average PSA 1447.57 ng/ mL and a PSA level greater than or equal to 100 ng/ ml in 50% of patients. Studies of Chen [6] and Eisenberger [7] have also reported higher median PSA with respectively 154 and 161 ng/ ml.
Forty - one (41) patients (58.6%) had Gleason score of 6 and 41.4% Gleason score greater than 6. On contrary, Chen [6] Eisenberger [7] and Botto [8] reported a predominance of little or undifferentiated cancers with respectively 53.1%, 57.6% and 64.1%. Also prostate cancers were detected in the metastatic phase in more than a half (82.4%). The study of Botto et al. [8] showed 69.2% of metastatic cancers and Fall et al. [3] 75%. This difference between our study and the study of Fall et al (two studies in the same department), could be explained by the fact that our patients had a more comprehensive staging.
Bone metastases, advanced age and androgen blockade are all independent factors contributing to the deterioration of bone mineral loss occurred thus promoting a bone event. It has been reported that nearly 95% of patients that died because of prostate cancer have bone metastases and, in most cases, with pathologic fracture, spinal cord compression, invasion and depletion of bone marrow [9]. According to the study by Smith on more than 12,000 patients, hormonal treatment multiplied by nine annual bone loss in older men with a 21% increased risk of fractures and 76% femoral neck fractures [7].
All the patients who had a biochemical recurrence after Radical Prostatectomy, had hormone therapy. This appears to be consistent with the recommendations [10]. Currently, the treatment of locally advanced prostate cancer requires a combination therapy combining a local control of cancer (radical prostatectomy or conformal radiotherapy radical prostatectomy followed by conformational radiotherapy) and hormone therapy [11]. However, we do not have that in our context radiotherapy and radical-prostatectomy hormone therapy protocol in locally advanced cancers is not yet anchored in our habits while an avenue of research promoted by several teams [10].
The choice of testicular pulpectomy is based on several factors:
• It is a most accepted surgical orchiectomy method which causes an obvious change in body scheme with significant psychological disorders [12]
• It is a more accessible method of therapy for most of our patients who cannot bear the cost of LHRH agonists
• Its action is faster than other castration methods. Testosterone is the castration levels at the end of the first time against 3 to 4 weeks for the LHRH agonist and 3 to 4 days for LHRH antagonists [12]. This quick action is important in our context where patients have bone pain or slow cord compression signs
• There are no flare-up effect as observed with LHRH agonists if the tumor mass is important.
The predominance of surgical castration has been reported in several countries including Nigeria [5] and Taiwan [6]. Admittedly the different time of action of surgical castration, agonists and LHRH antagonists are equally effective in terms of survival [8]. Concerning anti-androgen, bicalutamide was the most used. Its choice is justified by its superiority compared to Flutamide and Nilutamide, in terms of reduced side effects and in terms of survival [13]. Its use is a therapeutic option in cases of resistance during complete blockage with Flutamide as antiandrogen [13]. A reduction in mortality of 20% was reported when the complete androgen blockade is produced with the Bicalutamide compared with castration alone [14].
It is known that after hormone treatment, the PSA nadir is an important prognostic factor. Lower is the nadir, better is the survival [13-15-17]. The nadir was high compared to Gagnat et al. study [18] which found a median nadir of 0.48 ng/ ml. Several prognostic factors are recognized in patients treated with hormonal treatment for prostate cancer: Gleason score, the proportion of carrots invaded at the prostate biopsy, the PSA level at diagnosis, bone lesions [19].
The average time to reach the nadir (DAN) was short compared to the time in the literature. Indeed, Gagnat et al. [18] reported a median of 13.1 months DAN. Choueiri et al. [15] demonstrated for the first time that the DAN was a significant prognostic factor for overall survival in metastatic prostate cancer. Gagnat et al. [18] in their study confirmed that the DAN was a predictor of progression-free survival, overall survival and specific survival: higher the DAN was shorter the survival was.
Overall survival rates at 6 and 12 months were 46.2% and 23.1% respectively. This survival was short compared to those reported by other authors [8-18]. Indeed, Gagnat et al. [18] found an overall survival two years equal to 91.17% and Fall et al. [3] found 77.3 and 64.3% at 6 and 12 months respectively. This high mortality the first six months could be explained by important by the large tumor mass in our patients and extent of the alteration of the general condition as evidenced by the score of ECOG.
The average duration of overall survival was 6.9 months for metastatic cancers and 8.6 months for locally advanced cancers. This finding reinforces the recommendation of not to delay hormonal therapy in metastatic stage. Indeed, in advanced disease, Androgenic Suppression (AS) compared to immediate AS delayed the onset of clinical progression reduces complications related to disease progression, does not improve survival but increases slightly specific overall survival (5.5% decrease in risk of death at 10 years) [19]. For patients with stage T3-T4 N0-1 M0 inaccessible to local treatment (EORTC 30891), the immediate AS provides a benefit in terms of overall survival in patients less than 70 years, the PSA is > 20 ng/ ml or PSA doubling time <12 months [10].
The factors identified by Fall [3] to be associated with shorter survival were a high Gleason score and the existence of metastases at the time of androgenic suppression. The initial presence of bone metastases and poor tumor differentiation are universally associated with shorter survival [8-18]. The incidence of hot flashes is estimated between 60 and 80% [13-21]. None of our patients were reported unbearable hot flashes that would have required treatment. Some lifestyle changes such as stopping smoking and alcohol, wear light clothing, avoid the synthetic, avoid heat or intense cold, reduce coffee, were sometimes recommended [16] and decreased libido the erection problems are directly related to the decline in testosterone. The rate of erection dysfunction and decrease in libido that we reported are lower than those found by the study of Fall [3] (78.6 and 86%) and Potosky [17] (51 and 69%). This difference could be explained by the fact that the follow up in our study is not long enough and the fact that in our study there were 65% of patients who had more than 70 years. Two patients who wished to be treated, were put under type 5 phosphodiesterase inhibitor, and the results were satisfactory. Patients often came to consult in an advanced stage of the disease where anemia already existed. In those patients whose hemoglobin level was often below 7 g/ dl, they got blood transfusion before starting hormonal treatment. Fall et al. [3] reported anemia in 20% of these patients, but as they have proven we cannot exactly say that anemia is a consequence only of hormone therapy.
Conclusion
The diagnosis of prostate cancer to advanced stages is still common in our practice. Hormonal therapy still occupies a prominent place in the treatment of prostate cancer. Total PSA, its nadir, and time to reach the nadir (DNA) may be predictive factors for survival in metastatic prostate cancer. The mortality remains high in our regions, hence the need to find these cancers at an early stage in order to treat them adequately.
- Gomez SL, Le GM, Clarke CA, Glaser SL, France AM, West DW. Cancer incidence patterns in Koreans in the US and in Kangwha, South Korea. Cancer Causes & Control. 2003; 14: 167-174. https://goo.gl/pP6VR2
- Diao B, Fall B, Fall PA, Ze ondo C, Sow Y, Ndoye AK, et al. Prise en charge chirurgicale du cancer de la prostate à Dakar: analyse d’une série de 96 cas. Dakar Med. 2008; 53: 124-129.
- Fall B, Tengue K, Sow Y, Sarr A, Thiam A, Mohamed S, et al. Place de la pulpectomie bilatérale dans la suppression androgénique pour cancer de la prostate. Prog Urol. 2012; 17: 344-349. https://goo.gl/v5B88F
- Niang L, Ndoye M, Ouattara A, Jalloh M, Labou I, Thiam I, et al. Cancer de la prostate: quelle prise en charge au Sénégal? Prog Urol. 2013; 23: 36-41. https://goo.gl/WQcAZ8
- Oranusi CK, Nwofor AME. Evaluation of surgical castration for prostate cancer at Néwi: Issues regarding follow-up of cases. Tropical Journal of Medical Research. 2008; 12: 38-41.
- Chen C, Tzai T, Huang S, Wu H, Tai H, Chang Y, Pu Y. Clinical outcome of Taiwanese men with métastatic prostate cancer compared with other ethnic groups. Urology. 2008; 72: 1287-1292.
- Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998; 339: 1036-1042. https://goo.gl/rrqBim
- Botto H, Roupret M, Mathieu F, Richard F. Etude randomisée multicentrique comparant la castration médicale par triptoréline à la castration chirurgicale dans le traitement du cancer de la prostate localement avancé ou métastatique. Prog Urol. 2007; 17: 235-239. https://goo.gl/wBQSm9
- Rossi D, Beuzeboc P, Staerman F, Timsit MO, El Fegoun AB. Cancer de la prostate localement avancé et hormonothérapie. Prog Urol. 2010; 20: 68-71. https://goo.gl/AyVtXA
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017; 71: 630-642. https://goo.gl/vz1SRC
- Bellavita R, Massetti M, Abraha I, Lupattelli M, Mearini L, Falcinelli L, et al. Conformal postoperative radiotherapy in patients with positive resection margins and/or pT3-4 prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012; 84: 299-304. https://goo.gl/PUqqbp
- Fournier G, Valeri A, Mangin P, Cussenot O. Cancer de la prostate. Traitement. Annales d’urologie. 2004; 38: 225-258. https://goo.gl/ooda1g
- Koltz L. Hormone therapy for prostate cancer. European Journal of Cancer. 2007; 5: 415-417.
- Scher HI, Isaacs JT, et al. Prostate cancer. Clinical Oncology. 2émeedition. Churchill Livingston; New York: 2000. 1823-1884.
- Choueiri TK, Xie W, D’Amico AV, Ross RW, Hu JC, Pomerantz M, et al. Time to prostate specific antigen nadir in dependently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen deprivation therapy. Cancer. 2009; 115: 981-987. https://goo.gl/BU5v1S
- Miller JI, Ahmann FR. Treatment of castration-induced menopausal symptoms with low dose diethylstilbestrol in men with advanced prostate cancer. Urology. 1992; 40: 499-502. https://goo.gl/1WQP1z
- Potosky AL, Knopf K, Clegg LX, Albertsen PC, Stanford JL, Hamilton AS, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001; 19: 3750-3757. https://goo.gl/aG8khX
- Gagnat A, Larre S, Fromont G, Pires C, Doréa B, Irania J. La survie est associée au délai d’atteinte du PSA nadir (DAN) et au ratio DAN/valeur nadir après suppression androgénique pour cancer de prostate. Prog Urol. 2011; 21: 341-348. https://goo.gl/o8VnzH
- Rebillard X, Villers A, Ruffion A, Beuzeboc P, Soulie M, Richaud P, et al. Recommandations 2002 sur le cancer de prostate. Prog Urol. 2002; 12: 31-67.
- The Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol. 1997; 79: 235-246. https://goo.gl/hw8X1H
- Karling P, Hammar M, Varenhorst E. Prevalence and duration of hot flushes after surgical or medical castration in men with prostatic carcinoma. J Urol. 1994; 152: 1170-1173. https://goo.gl/75pz3N


Sign up for Article Alerts