Place of Man in the Hypofertile Couple in Senegal
Yoro Diallo1*, Mama Sy Diallo2, Saint Charles Kouka1, Thierno Diallo1, Néné Mariama Sow1, Modou Diop Ndiaye1, Cheikh Diop1, Mehdi Daher1, Ramatoulaye Ly1, Seydou Diaw1 and Cheikna Sylla1
1Faculty of Health Sciences, University of Thiès
1Department of Histology Embryology and Cytogenetics Hospital Aristide le Dantec
*Address for Correspondence: Yoro Diallo, UFR Health Sciences, University of Thiès - BP 967 Thiès, Senegal, Tel: +221-775-525077; E-mail: yorodiallo@hotmail.com
Submitted: 05 March 2019; Approved: 06 March 2019; Published: 07 March 2019
Citation this article: Diallo Y, Diallo MS, Kouka SC, Diallo T, Sow NM, et al. Place of Man in the Hypofertile Couple in Senegal. Am J Urol Res. 2019;4(1): 001-007.
Copyright: © 2019 Diallo Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Hypofertility; Couple ; Spermogram; Varicocele
Download Fulltext PDF
Introduction: The purpose of our work is to study the epidemiological and clinical aspects of the hypofertile couple, to establish the human role in the infertile couple in our context.
Patients and methods: This is a multicenter and prospective study of couples consulting for subfertility in the regions of Dakar, Thiès, Kaolack and Touba. It took place over a period of 12 months from October 1, 2015 to September 30, 2016.
Results: Our study focused on 301 couples. The average age was 30 years for women and 40 years for men with extremes of 16 to 50 years and 20 to 69 years respectively. Subfertility was more common in the age group 30-39 years for females and 40-49 years for males. Men in school were higher than women with 84.3% and 55% respectively. Primary hypofertility was the most representative at 72% compared to 28% for secondary infertility. In primary infertility, the mean age was 39.3 +/ - 8 years for males and 29.2 +/ - 6 years for females. The average length of the couple’s desire to be a child was just over 8 years (8.38) with extremes of 1 and 25 years. Varicocele was found in 140 cases or 50.3% of cases. Asthenospermia was the most common abnormality, accounting for 55.9% of cases followed by oligospermia with a rate of 51.8% versus 39% of cases with necrospermia. In humans, a rise in the rate of FHS was found in 69.6% of cases and LH in 30.4%. For 40 cases of azoospermia, the FSH level was dosed 18 times. The spermogram of control was abnormal in 95% of the cases. We noted a worsening of the spermogram in 50% of the cases. Five cases of success with live birth after natural fertilization were recorded. Conclusion: the responsibility of the man is proven, a multidisciplinary collaboration is fundamental.
Introduction
Hypofertility is the inability of a couple to conceive a child during a year of living together without contraception [1]. It is becoming more common and a public health problem. The particularity of the sub-Saharan regions of Africa, particularly in Senegal, lies in the fact that the prevalence is underestimated and the woman is seen as the main if not the only person responsible for conjugal infertility. The diagnosis of subfertility requires a rigorous approach based on good anatomo-physiological and clinical knowledge. The spermogram constitutes an essential examination in the establishment of the diagnosis and the appreciation of a potential cause. The aim of our work is to study the epidemiological and clinical aspects of the infertile couple, to establish the part of the man in the infertile couple and to evaluate the current state of care of the infertile couple in our context.
Patients and Methods
This is a multicenter and prospective study of couples consulting for subfertility in Dakar, Thiès, Kaolack and Touba regions. It took place over a period of 12 months from 1 October 2014 to 30 September 2015. In Dakar, these included: Uro-Andrology, Gyneco-Obstetrics at Grand Yoff General Hospital (HOGGY), Uro-Andrology Department at Dakar Hospital (HPD), Center of Gerontology and Geriatrics of Ouakam (CGGO) and the clinic of the Senegalese Association for Family Welfare (ASBEF). In the other regions, these were Uro-Andrology departments of: Saint Jean de Dieu Hospital in Thiès (HSJD), Mbour Public Health Establishment (EPSM), El Hadj Ibrahima Regional Hospital Niass de Kaolack (HREIN) and the Matlaboul Fawzayni Hospital of Touba (HMFT).
Our study focused on 301 couples who came to consult for subfertility. During the survey, each member of the couple was well informed and volunteered to participate in our study. The questionnaire validated jointly by the Interafrican Group for Research, Research and Application on Fertility (GIERAF) and the French Language Andrology Society (SALF), was our working support.
The inclusion criteria concerned any couple who consulted for a subfertility lasting more than or equal to 1 year and who gave their consent to the study. Couples who have been lost or refused to participate have been excluded.
The parameters evaluated were: Origin of couples, age, occupation, level of education, height, weight, body mass index, type of subfertility, length of life in a couple, the existence or not of sexual disorders, habits and lifestyle (smoking, alcoholism), occupational risk factors, medical history, and family history of subfertility. Data from the physical examination of man and woman, spermogram results, ultrasound, hysterosalpingography, plasma hormonal assays (FSH, LH, testosterone). Treatment initiated and follow-up.
Results
Nearly half of our study population was recruited from the HOGGY urology and gynecopsy services (coordinating center) with 45% or 137 couples, followed by Thies centers with 21%. The centers of Mbour and Touba represent respectively 8% and 7% (Table 1).
The average age was 30 years for women and 40 years for men with extremes of 16 to 50 years and 20 to 69 years respectively. Hypofertility was more frequent in the age group 30-39 years for females and 40-49 years for males (Table 2).
In men, the most found professional categories were workers with 38% followed by civil servants 32% of cases. The traders were estimated at 26% against only 3% for the nursing staff and 1% for the students.
While among women, housewives were estimated at 71% against only 10% for civil servants, 7% for female students and 6% for women traders.
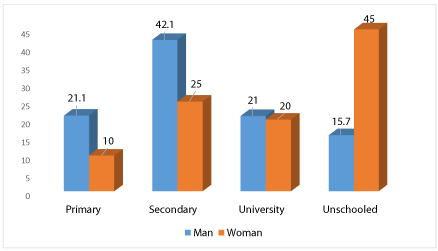
Men in school were higher than women at 84.3% and 55% respectively (Figure 1).
The majority of men, 78.8%, had a BMI between 18.5 and 24.9. In 17.9%, men had a BMI between 25 and 29.9 and in 2.5% of cases BMI was greater than 30. Only 1% of men had a BMI less than 18.5. In women, the majority (74.6%) had a BMI between 18.5 and 29.9. But in 22.9% of cases women had a BMI less than 18.5. Only 2.5% of women had a BMI between 25 and 29.9. No woman had a BMI greater than 30.
Primary hypofertility was the most representative at 72% compared to 28% for secondary infertility. In secondary infertility, the last child of the couples was at least 5 years old in more than 50% of the cases. The predominance of primary infertility was found in all centers individually.
In primary infertility, the mean age was 39.3 +/ - 8 years for males and 29.2 +/ - 6 years for females. But in secondary infertility we find a higher average age for both men and women with respectively 44.6 years and 33.1 years (Table 3).
The average length of the couple’s desire to be a child was just over 8 years (8.38) with extremes of 1 and 25 years. There was a predominance during the first 6 years and a peak between 2 and 5 years.
The average common life of couples was 9 years with extremes of 1 and 25 years.
An abnormal erection was found in 3.65% (n = 11) and abnormal ejaculation in 3.32% (n = 10). The association of the two abnormalities was found in 2% of the total number (6 cases).
In more than half (60.5%) cases the number sexual relation per week were 3 to 4. But we met a significant share or 32.6% of couples who practice less sex during their living together versus only 7% who exceeded 4 sexual intercourse per week.
The smoking rate was estimated at 22.9% (n = 69) compared to only 2.7% of alcohol consumption.
We encountered 9 cases of occupational exposure, a rate of about 3%. The use of certain products was reported. It was 2 cases concerning pesticides, 2 cases for sulfur, 2 cases for unspecified pesticides and for other heavy metals and unspecified hydrocarbons. One case was reported for each of the following products: steel, copper.
No cases of radiotherapy or chemotherapy were encountered. No case of medical treatment with precision has been reported. In contrast, 48.8% of our workforce had started traditional drug therapy. The duration of traditional treatment was 1 to 6 months in 68.9% of cases. In 18% of the cases our couples followed this treatment during 7 to 12 months. Only 3.4% did not reach 1 month of treatment.
In our human study, 11 cases of a history of mumps in childhood were found at 3.7%. In about 2% of cases we found a urinary tract infection. A notion of sexually transmitted diseases accounted for 1.7% of cases. Two cases of pulmonary and vertebral tuberculosis were found.
Urgical antecedents
Varicocele cure largely dominated with an estimate at 26.5% of the total number, ie 80 cases. Stock market trauma was estimated at 2.32% or 7cas. In addition we found 4 cases of cryptorchidie cure, 1 case of inguinal hernia repair and 1 case of torsion cure of the testicle.
Gynecological obstetrical history
Some of the women we met during the survey had at least one previous pregnancy in 35.6%, previous birth in 27.5%. Only 2 women confided to us to have made a voluntary termination of pregnancy is a rate of 0.9% against 9.9% of cases spontaneous miscarriages. In about 12% of cases, women had a dysovulation-type cycle disorder.
In women, we found a family history of infertility among brothers in 57% of cases, uncles 26%, sisters 13% against only 4% in aunts.
In men, a family history of infertility was found in 57% of the cases concerning the brothers. And 26% of cases concerned uncles versus 13% of sisters and 4% of aunts.
Family history of infertility (male or female) is 3 times more common in primary infertility.
During the physical examination, the search for varicocele was performed in 278 infertile men at a rate of 92.3%. Thus 140 cases of varicocele were found, a rate of 50.3%. The remaining 49.7% did not have varicoceles.
The bilateral form of varicocele was the most representative, about 88.6% of the cases, compared to 11.4% for the unilateral form.
Varicocele was more common in primary infertility with 72.9% versus only 27.1% in secondary infertility
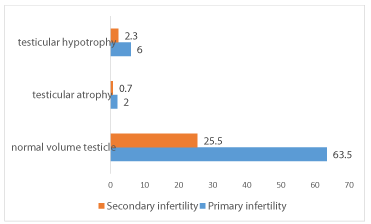
A decrease in testicular volume was found in about 11% of infertile men with 8.3% of cases of hypotrophy against 2.7% of cases of testicular atrophy. Thus primary infertility was accompanied by testicular hypotrophy in 6% of cases and testicular atrophy in 2%. Secondary infertility is associated with testicular hypotrophy in only 2.3% and testicular atrophy in 0.7% of cases. Only one of our patients had a single testicle. The decrease in testicular volume was associated with varicocele in 63.6% of cases (Figure 2).
In addition, 6 cases of hydrocele were found at a rate of about 2%, compared to 1 case of epididymal cyst. No abnormalities of the vas deferens were found. In addition, the search for gynecomastia was negative.
The WHO 2011 standards were our benchmarks for the analysis of spermogram results. Thus, during our study, all the men involved had a spermogram and that was abnormal in 86.4% of the 301 cases.
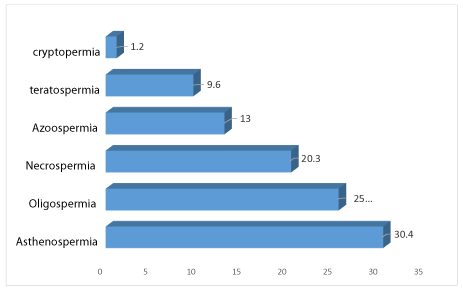
Asthrenospermia was the most common abnormality (30.4%), followed by oligospermia (25.5% versus 20.3% necrospermia and 13 azoospermia (Figure 3).
In 6.6% of cases, sperm volume was less than 1.5 ml. In 87.9% the volume was between 1.5 to 6 ml against only 3.7% of the cases where the volume was greater than 6 ml. Hypospermia defined by a sperm volume of less than 1.5 ml was estimated at 3.6% after 40 years against 3% between 30 and 40 years. And semen volume was normal in all patients under 30 years of age.
A concentration greater than 15 million per ml was estimated at 38.9%, against 24.6% of cases where the concentration was between 5 and 15 million per ml. It was greater than 0 and less than 5 million per ml in 17.3% whereas azoospermia was estimated at 19.3% (58 cases).
The sperm count was normal in 47.8% in the absence of varicocele compared to only 28.6% of normal cases in the case of varicocele. Oligospermia was more common in cases of varicocele with a rate of 52.2% compared with 33% in the absence of varicocele. We found the same number of cases of azoospermia, 19.3% in both groups, with or without varicocele.
A vitality greater than 58% was found in the majority of the 243 cases (60.9%) against 39% of cases where the vitality was lower 58% corresponding to the necrospermia of which the 19.8% more severe (vitality lower than 30% ).
Necrospermia was found in the majority of patients with an infection with a rate of 55% against only 45% of cases where the vitality was normal.
Sperm motility was normal in 44% of cases (mobility greater than 40%). A mobility of 20 to 40% was estimated at 32.1% while 23.9% of cases mobility was less than 20%.
In our smoking patients, normal mobility spermatozoa (greater than 40%) was 26.9%. Asthenospermia was found to be 73.1%, corresponding to a mobility of less than 40%.
Sperm morphology was normal in 84%. In 8.2% of the cases the normal forms were less than 15% compared to 7.8% for the normal forms of 16 to 30%.
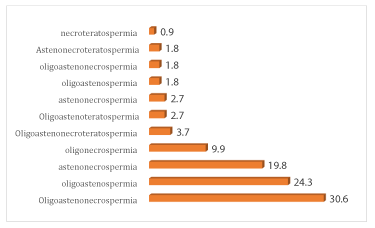
Of the associations of sperm abnormalities, oligoazeno-necrospermia was the most common with a rate of 30.6% followed by oligoasthenospermia in 24.3%, asthenonecrospermia in 19.8% and oligonecrospermia in 9.9% of cases. Oligo-astheno-necro-teratospemia was found in 3.6% of cases and oligo-astheno-teratospermia in 2.4%. The least common association of abnormalities was necroteratospermia with 0.9% of cases.
Spermoculture was performed in 29 patients, or 9.33% of the total population. In 6 patients, it allowed to objectify an infection with Staphylococcus aureus, Ureaplasma urealyticum, Chlamydia trachomatis and gram negative cocci.
Fellowship ultrasonography confirmed the presence of varicocele and abnormalities of testicular volume and epididymis. Moreover, the ultrasound had found in one of our 2 spermatocele solidarity on the left.
In the woman, the ultrasound evoked 6 cases of isolated ovarian cysts, 6 cases of ovarian polycystoses and 1 case of bilateral ovarian dystrophy.
Women who had benefited from hysterosalpingography were estimated at 64.1% or 193 of the total number. Tubes were normal in 89.1% of cases. Thus tubal obstruction was found in about 10.9% of cases with a predominance of the bilateral form is 6.8% against 4.1% for unilateral tubal obstruction.
In humans, a rise in the rate of FHS was found in 69.6% of cases and LH in 30.4%. Prolactinemia and testosterone were normal for the 6 and 18 cases respectively.
For 40 cases of azoospermia, the FSH level was dosed 18 times. This hormone was high in 17.5% of cases; which was in favor of non-obstructive azoospermia compared to 27.5% of cases where FSH levels returned to normal.
Testicular origin of the spermatogenic disorder was found in 3 cases of the 6 performed testicular biopsies.
During each type of treatment the psychological approach was appropriate.
During drug treatment, the use of vitamin E and/ or B was common. HCG beta injections were made for suspected cases of Klinefelter syndrome.
During the course of the surgical treatment, the cure of varicocele occupied an important place with a rate of 26,5% of the cases.
Some of the women with bilateral tubal obstruction had had insufflation.
The evolution of the spermatic parameters was marked by an aggravation or a decrease of the anomalies.
The spermogram of control was abnormal in 95% of the 122 cases performed. We noted a worsening of the spermogram in 50% of the cases. The check was carried out at least 3 months after the first spermogram after treatment or not. In addition 5 cases of success with live birth after natural fertilization, we had been notified.
Discussion
Hypofertility is a major problem in a couple’s life. In our context, there are few studies that take into account men and women at the same time [2]. The majority of couples who have consulted for spousal sub-fertility are relatively young. In women, the average age is 30 years with a notable variation depending on the type of subfertility. While in men, the average age of consultation is higher in both primary and secondary human subfertility, our results are much higher than those of Rodet F. [3] who found an average of age of 33.2 years. Ndoye M et al. [4] and Hounnassou [5] found an average age comparable to ours with 39 years and 37 years respectively. In women, the age group of 20-29 years represents a significant share with 43.7%. These age variations could be explained by the constitutional nature of the couple where the woman is usually younger. Age is a factor to consider, if we know that the younger the couple, the better the chances of conceiving. Regarding humans, some authors have demonstrated a progressive decrease in semen volume in relation to age [5].
In our study, the responsibility for subfertility is shared. Infertility is reported to be 40% male, 23% female and 37% mixed [2]. Our results on shared responsibility approach those of several studies. Like Hounnassou [5], and Fournols LJ [6] who found a male responsibility estimated at 30%, that of the woman at 30%. Agarwal A. [7] found a greater female responsibility of around 73.2%, compared to only 2.1% for men and 18.6% for couples. This finding demonstrates the proven role of the man in the subfertility of the couple contrary to the empirical beliefs in our context where only the woman is responsible for the failure of the concession. As a result, she suffers some injustices including unsubstantiated accusations of the beautiful family and some of them justify it to look for a second wife.
Changes in weight can have a negative effect on reproductive potential. It is in this sense that Christin S, et al. [8] mentioned that obesity has a deleterious role in fertility, according to him, more obesity is important more asthenospermia is pronounced. While Freour T [9] already argued that women of underweight or overweight can experience menstrual irregularities compromising their fertility. Puech F. [10], referring to CNGOF’s recommendations, ranked obesity among the factors of subfertility. These authors go so far as to show that a Body Mass Index (BMI) ≥ 35 would halve the chances of pregnancy. All these data support our results. However, the mechanism of sperm quality alteration under the influence of obesity is not fully understood.
Some professions may have some degree of harm to the conditions of good reproductive function. This can be explained by the “aggressive” nature of these on spermatogenesis including prolonged sitting positions that have a circulatory blood deficit and exposure to high temperatures. A study conducted in Mali, noted a higher frequency of traders with 29% versus 26% in our series [11].
The use of certain products has been reported by several authors. Chemicals have harmful effects on fertility [12]. In the United States, according to a study among women consulting for subfertility, the use of herbicides or fungicides has been identified as a risk factor while the fact of residing in rural areas is recognized as a protective factor of fertility [13].These authors noted that pesticides are a long-term potential danger to reproductive health [14].
The rate of non-enrollment of women (45%) is 3 times higher than that of men. We have the same observations in the general population. The authors do not find a significant impact on the follow-up of patients because they are motivated and sometimes respect better the therapeutic guidelines [15,16]. There may be a selection bias, as educated women at higher levels tend to go to private clinics to increase their chances of designing new therapeutic methods.
Smoking is an important etiological factor in infertility because it has a direct effect on spermatogenesis and leads to severe sperm alterations [1,17]. It reduces the quality and the number of spermatozoa [18]. The mobility of spermatozoa is reduced in our smoking patients with asthenospermia found in 73.1%. Laudat A. [19], when he found 30% of cases of smoking among men in Mali. It is therefore a real danger for male fertility. A notion of alcohol consumption is found in 2.7% of cases; rate a little lower than that found by Ndoye M. [4] or 3.5%. It promotes erectile dysfunction and causes an alteration in the number and quality of spermatozoa.
In our context, traditional treatment is one of the first remedies for the management of infertility. It may affect the psychological aspect of conjugal infertility, but the problem remains to improve the quality of sperm of patients [20].
The notion of infertility in the family history in our study denotes the probably genetic nature of it. However, few studies are devoted to the familial aspect and the hereditary chromosome abnormalities that can explain these cases. In men, a family history of hypofertility is found in 57% of cases concerning brothers, 26% for uncles versus 13% for sisters and 4% for aunts. Family history of infertility (male or female) is 3 times more common in primary infertility. Would this be an argument going in the direction of better considering the genetic causes in conjugal hypofertility? It is likely that a number of genetic cases are missed by screening for mutations in the CFTR gene and looking for Y chromosome microdeletions [19].
We have a clear predominance of primary infertility. This result is similar to that found by Ndoye M. [4] and Niang L. [17] with respectively 66.5% and 62%. This trend has persisted over time based on an analysis of infertility in sub-Saharan Africa [21]. This reflects, of course, the importance that primary infertiles give to this scourge, which pushes them to come to consult as soon as possible.
Infections that are not or poorly treated also have a great deal of responsibility for the couple’s infertility. Several causes have been reported in our series. All authors are unanimous on the deleterious role of germs on spermatogenesis [22]. The most commonly encountered germs are: Ureaplasma urealyticum, Mycoplasma hominis and Neisseria gonorrhoeae [20,23].
In a study conducted in Senegal, varicocele accounted for 46.5% of the aetiologies of conjugal infertility. This same finding was underlined by Ndoye M. [4] with a rate approaching 65.43%. In France, a lower rate of 40% was found [24]. Varicocele is one of the main causes of primary infertility about 72.9% compared to 27.1% for secondary infertility. The sperm count is normal in 47.8% in the absence of varicocele compared to only 28.6% of normal cases in case of varicocele. Oligospermia is more common in cases of varicocele with a rate of 52.2% compared to 33% in the absence of varicocele. In varicocele carriers, we can see an increase in tapered and irregular head shapes and a decrease in the concentration and mobility of spermatozoa [21].
A tubal obstruction is found in a non-negligible population in our study. There is a clear predominance of bilateral form. The etiologies are essentially of order ovulatory, cervical, tubal and endometriosis. A study carried out in France, found a high rate of tubal obstruction or 52.7% of cases [25].
In the spermogram, asthenospermia is the most frequent isolated anomaly (55.9% of cases followed by oligospermia with 51.8%). Azoospermia was found in 19% of cases. Our results are similar to those found by Shreffler KM [26], where 77% of infertile men had simple or complex cyto-spermiological abnormalities. Among the associations of anomalies with the spermogram, various possibilities were found. The association of abnormalities least frequent in our study is necro-teratozoospermia. While in the literature, oligo-astheno-necro-teratospermia and Oligo-Astheno-Teratospermia (OATS) were the most common [27].
In our study, we found a rise in the rate of FHS in 69.6% and LH in 30.4% of cases. These results are less compared to those of Polis CB. [28] found a rise in FSH levels in 8 patients with azoospermia (more than 48%). This disparity is explained by the low use of hormonal assays that are not available in some areas. In all cases, it is necessary to recognize the importance of plasma hormonal assays in the central or peripheral aetiological orientation of azoospermia.
The therapeutic follow-up of our couples is ensured at the level of each center by urologists or gynecologists. However, in all cases, the treatment of conjugal infertility requires multidisciplinary management [29]. The treatment of choice is represented by AMP. Indeed, the probability of getting pregnant with these new therapeutic methods is more important. Consultation with the couple is essential to determine the prognosis and the different possibilities offered by assisted procreation. To optimize the management, a collaboration between the various stakeholders ie urologists, gynecologists and biologists should be the first link to consolidate.
Conclusion
The responsibility of the man in the conjugal infertility is proved. The main contributing factor is represented by varicocele. A joint consultaftion at the same time and a collaboration between gynecologist and urologist is interesting to the extent that the same level of information is a prerequisite in a good approach for efficient management. Raising the technical platform with better access to assisted procreation methods would make it possible to optimize the chances for couples wanting children.
- Gunes S, Arslan MA, Hekim GNT, Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet. 2016; 33: 553-569. https://goo.gl/RwWhKA
- Tarin JJ, Perez AG, Hamatani T, Cano A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod Biol Endocrinol. 2015; 13: 1-11. https://goo.gl/uFmnLY
- Rodet F, Huijbregts L, Vilanueva C, Villoing L, Jacquier S, Roux DN. Le couple Kisspeptine/ GPR54, un acteur majeur de la regulation neuroendocrine de la reproduction. Médecine de la Reproduction. Gynécologie Endocrinologie. 2008; 10: 123-130. https://goo.gl/Rh3QDq
- Ndoye M, Niang L, Labou I, Jalloh M, Kane R, Diaw J, et al. Azoospermie au Sénégal: quelle prise en charge à l’heure de l’ICSI?. Andrologie. 2008; 18: 206-209. https://goo.gl/MuX6Gy
- Hounnassou PP, Gandaho KI, Avakoudjo JDG, Hodonou PZR, Vodounou AA, Akpo EC. Profil spermiologique des hommes consultant pour infertilité à Cotonou. Rev Afr Uro Andro. 2013; 1: 22-26. https://goo.gl/KyVhwh
- Fournols LJ, Patrat C. Infertilité masculine – le sperme: du bon au mauvais. Annale d’Endocrinologie. 2014; 75: 258-262. https://goo.gl/7GXFHk
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reproductive Biology and Endocrinology. 2015; 13: 2-9. https://goo.gl/cneip1
- Christin MS. À la recherche d’une étiologie génétique de l’infertilité féminine. Médecine de la Reproduction. Gynécologie Endocrinologie. 2010; 12: 12-7. https://goo.gl/7fM2z5
- Freour T, Delvigne A, Barrière P. L’exploration de l’homme du couple infécond. Journal de Gynécologie Obstétrique et Biologie de la Reproduction. 2010; 39: 45-52. https://goo.gl/dG2hs5
- Huyghe E, Izard V, Rigot JM, Pariente JL, tostain J. Evaluation de l’homme infertile: recommandations AFU 2007. Prog Urol. 2008; 18: 95-101. https://goo.gl/NDbiHc
- Shaeer OKZ. "La vasovasostomie de Shaeer”: comparaison de la voie intra-péritonéale et voie extra-péritonéale en cas d’obstruction inguinale du canal déférent. Andrologie. 2006; 16: 53-55. https://goo.gl/K8kVjC
- Loposso NKM, Punga Maole AML, Moningo MD, Tschitala BD. Oligoasthenoteratospermia (oats) in hospital milieu of Kinshasa: a retrospective analysis. Ann Afr Med. 2010; 3: 533-541. https://goo.gl/BWDTo9
- Fauregeron RI, Christin MS. Mutations des récepteurs des gonadotrophines. Médecine de la reproduction. 2005; 7: 91-99. https://goo.gl/xs41if
- Multigner L. Effets retardés des pesticides sur la santé humaine. Environnement, Risques & Sante. 2005; 4: 187-194. https://goo.gl/hCgdVN
- Faure C, Dupont C, Sermondade N, Levy R. Antioxidants and male infertility. Médecine de la Reproduction. Gynécologie Endocrinologie. 2011; 13: 275-283.
- Keskes LA, Gdoura R, Bouzid F, Bensalah FZ, Sellami D, Hakim HK. Retentissement de l’infection génitale à chlamydia trachomatis sur le sperme chez les hommes consultant pour infertilité du couple. Andrologie. 1998; 8: 25-35. https://goo.gl/C19dJU
- Niang L, Labou I, Ali O, Jalloh M, Kane R, Ndoye M, et al. Varicocèle primitive et infertilité: evaluation postopératoire des paramètres du spermogramme et de la fécondité. African Journal of Urology. 2007; 13: 214-219.
- Willem O, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008; 14: 605-621. https://goo.gl/oJEf4H
- Laudat A, Lecourbe K, Palluel AM. Le tabac: un inducteur potentiel de peroxydation lipidique membranaire du spermatozoïde humain ? Ann Biol Clin. 2004; 62: 681-686. https://goo.gl/Z6uCNY
- Imudia AN, Detti L, Puscheck EE, Yellian FD, Diamond MP. The prevalence of ureaplasma urealyticum, mycoplasma hominis, chlamydia trachomatis and Neisseria gonorrhoeae infections, and the rubella status of patients undergoing an initial infertility evaluation. J Assist Reprod Genet. 2008; 25: 43-46. https://goo.gl/J9uqu7
- Niang L, Ndoye M, Labou I, Jalloh M, Guèye SM. Profil épidémiologique et clinique de l’infertilité masculine à l’hopital général de Grand Yoff, Sénégal: à propos de 492 cas. Andrologie. 2009; 19: 103-107.
- Putin C, Bianchetti S, Lornage J. Incidence of the infection of the genital tract and accessory glands on the male fertility. Médecine de la Reproduction. Gynécologie Endocrinologie. 2010; 12: 233-241.
- Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics. 2018; 73: 364-370. https://goo.gl/L4qkfj
- Nevoux P, Mitchell V, Chevallier D, Rigot JM, Marcelli F. Varicocele repair: does it still have a role in infertility treatment?. Curr Opin Obstet Gynecol. 2011; 23: 151-157. https://goo.gl/4t7KXh
- Hanson B, Johnstone E, Dorais J, Silver C, Peterson M, Hotaling J. Female infertility, infertility-associated diagnoses, and comorbidites: a review. J Assist Reprod Genet. 2017; 34: 167-177. https://goo.gl/ZEW41p
- Shreffler KM, Greil AL, McQuillan J. Responding to infertility: lessons from a growing body of research and suggested guidelines for pratice. Fam relat. 2017; 66: 644-658. https://goo.gl/TWZexz
- Abarikwu SO. Causes and risk factors for male-factor infertility in Nigeria: a review. Afr J Reprod Health. 2013; 17:150-166. https://goo.gl/vx7Nyx
- Polis CB, Cox CM, Tunçalp Ö, McLain AC, Thoma ME. Estimating infertility prevalence in low-to-middle-income countries: an application of a current duration approach to demographic and health survey data. Hum Reprod. 2017; 32: 1064-1074. https://goo.gl/fMeRkG
- Marcelli F, Robin G, Rigot JM. Treatment of male infertility. Prog Urol. 2009; 19: 260-264.

Sign up for Article Alerts