A Brief Study of the Correlation of Urine D-ribose with MMSE Scores of Patients with Alzheimer’s Disease and Cognitively Normal Participants
Jihui Lyu1#, Le-Xiang Yu1#, Ying-Ge He1, Yan Wei1, Li-Ling Dong3, Shou-Zi Zhang2, Yi Ma1 and Rong-Qiao He1*
1State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, University of Chinese Academy of Sciences
2Center for Cognitive Disorders, Beijing Geriatric Hospital
3Neurology department, Peking Union Medical College Hospital
4The Medical Examination Center, Beijing Geriatric Hospital
#Equal contribution to this work
*Address for Correspondence: Rong-Qiao He, State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Chaoyang District, Beijing 100101, China, Tel: +010-648-89876; E-mail: herq@ibp.ac.cn
Submitted: 28 April 2019; Approved: 28 May 2019; Published: 29 May 2019
Citation this article: Lyu J, Yu LX, He YG, Wei1 Y, Rong-Qiao He, et al. A Brief Study of the Correlation of Urine D-ribose with MMSE Scores of Patients with Alzheimer’s Disease and Cognitively Normal Participants. Am J Urol Res. 2019;4(1): 018-023.
Copyright: © 2019 Lyu J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: D-ribose; Alzheimer’s disease; Cognitive impairment; Glycation; MMSE
Download Fulltext PDF
Glycation plays a role as risk factor in Alzheimer’s Disease (AD) and D-ribose is one of the important contributors to protein glycation. Here, we recruited 93 participants (49 AD patients, 44 cognitively normal participants) and measured their cognitive abilities with Mini-Mental State Examination (MMSE), D-ribose and formaldehyde levels in the morning urine. Urine D-ribose levels of AD patients (especially women) were significantly higher than those of cognitively normal participants. Like formaldehyde, D-ribose levels were negatively correlated with MMSE scores in all participants. Unlike formaldehyde, D-ribose levels still showed the negative correlation in cognitively normal participants. These data may provide a novel clue to study AD.
Abbreviations
MMSE: Mini-Mental State Examination; AD: Alzheimer’s Disease
Introduction
Diabetes is considered as an important risk factor for Alzheimer’s Disease (AD) [1-3], which is called the third diabetes [4]. According to a population-based cohort study, uncontrolled diabetes increases the risk of AD [5]. Non-enzymatic glycation has been found to be involved in neurodegenerative diseases like AD [6], and thus increased attention is paid to the role of glycation in AD pathogenesis [7]. Protein glycation is a cause for complications in diabetes mellitus [8-10]. A recent study has showed that patients with diabetes suffer from abnormal metabolism of not only D-glucose but also D-ribose [11]. Thus, the role of D-ribose in the glycation and age-related cognitive impairment is necessary to be investigated. D-ribose, which is naturally found in human body, participates in numerous biochemical processes, especially in energy production [12,13]. D-ribose is much more reactive with protein than D-glucose such as glycation of neuronal Tau [14] and α-synuclein [15] under the same experimental conditions [16,17], acting as one of the major contributors to the Glycation Of Serum Protein (GSP) [18] and Hemoglobin (HbA1c) [19]. Intraperitoneal injection of D-ribose leads to elevation of formaldehyde levels in mouse brain [20]. Ribosylated Tau protein forms globular aggregation that is cytotoxic to neuronal cells [14]. Excess and long-term administration of D-ribose induces cognitive impairment followed by amyloid-β deposition and Tau hyperphosphorylation in mouse hippocampus and cortex [21]. Although effects of endogenous formaldehyde and D-ribose on cognitive impairment has been studied in this laboratory [21-24], the relationship between cognitive ability and D-ribose has not been clinically investigated. Here, we aim to explore the correlation between endogenous D-ribose levels and MMSE scores, compared to those between endogenous formaldehyde levels and MMSE scores.
Materials and Methods
Participants
All participants were enrolled between August 2015 and April 2018 at the Center for Cognitive Disorders, or the Medical Examination Center of Beijing Geriatric Hospital, and Neurology department of Peking Union Medical College Hospital, China. The inclusion criteria for AD patients were as follows: (a) 55 years old and older, (b) with diagnosis of probable AD based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria. The inclusion criteria for cognitively normal participants were as follows: (a) 55 years old and older, (b) with the Clinical Dementia Rating scale (CDR) = 0 (no dementia) [25]. Exclusion criteria included: with diabetes or hyperglycemia, with life threatening disease, with severe mental illness, unable to receive the assessments for any reason, or substance abuse. Participants were enrolled by dementia specialists in the research team. The diagnosis of AD was based on a detailed medical history, comprehensive physical examination, neuropsychological assessments, relevant blood test, and brain imaging examination (CT or MRI, and PET when necessary) and according to NINCDS-ADRDA criteria. This study was conducted in accordance with guidelines on human subject research and approved by the ethical review committee of the Beijing Geriatric Hospital (BJLNYY-2018-011) and by the ethics committee of the Institute of Biophysics, Chinese Academy of Sciences (2014-HRQ-1).
Measurements
All participants were assessed with the CDR [25] and the Chinese version of Mini Mental State Examination (MMSE) [26]. The CDR is a rating scale for the clinician to characterize the degree of severity of dementia (0 = no dementia, 0.5 or 1 = mild dementia, 2 = moderate dementia, 3 = severe dementia). The MMSE is a 30-point questionnaire which is used to measure cognitive impairments. It examines functions of attention, calculation, recall, language, ability to follow simple commands and orientation.
Urine D-ribose
Analyses of urine D-ribose were performed in a double-blind manner by the Center of Cognitive Disorders (Center for Cognitive Disorders, Beijing Geriatric Hospital, China), and Neurology department (Peking Union Medical College Hospital, Beijing, China). Urine D-ribose were measured as described previously [11]. A 1.0 ml urine sample (thawed at 4° C) was centrifuged (12,000 rpm, 4° C, and 30 min). A 0.4 ml of the supernatant was mixed with 0.6 ml 4-(3-methyl-5-oxo-2-pyrazolin-1-yl) benzoic acid (MOPBA, final concentration 150 mM, 250 mM NaOH (Cas: 20070319, Guoyao, China) in 50% methanol (Cas: A452-4, ThermoFisher, USA)-water solution) (Cas: 197556, Sigma, USA). Samples were centrifuged (12,000 rpm, 4° C, and 10 min) and then reacted in a 70° C water bath for 90 min; this was followed by centrifugation (12,000 rpm, 4° C, and 10 min). The mixture was acidified by addition of 150 μl of aqueous 2 M HCl (Cas: 80070591, Beijingshiji, China) solution to precipitate the excess MOPBA. The mixture was vortexed and centrifuged (12,000 rpm, 4° C, and 10 min) and then filtered (0.22 μm). 20 μl of the solution was then subjected to high-performance liquid chromatography (HPLC) (LC-20A, Shimadzu, Japan)
Urine formaldehyde
Analyses of urine formaldehyde were performed in a double-blind manner by the Center of Cognitive Disorders (Center for Cognitive Disorders, Beijing Geriatric Hospital, China) and Neurology department (Peking Union Medical College Hospital, Beijing, China). Urine formaldehyde were measured as described previously [27]. A 1.0 ml urine sample (thawed at 4° C) was centrifuged (12,000 rpm, 4° C, and 30 min). A 0.4 ml of the supernatant was mixed with 0.4 ml acetonitrile (Cas: A998-4, ThermoFisher, USA), 0.1ml TCA (20%, w/v, in water) (Cas: 80132618, Guoyao, China) and 0.1 ml 2,4-dinitrophenylhydrazone (1g/L) (Cas: 550626, Beijingshiji, China). Samples were centrifuged (12,000 rpm, 4° C, and 10 min) and then reacted in a 60° C water bath for 30 min; this was followed by centrifugation (12,000 rpm, 4° C, and 10 min) and then filtered (0.22 μm). 20 μl of the solution was then subjected to High-Performance Liquid Chromatography (HPLC).
Statistical analysis
The significances between two groups were calculated with two-sided unpaired Student t-tests and Person Chi-square test. All values are reported as the means ± Standard Error of the Means (SEM), a difference of P < 0.05 is considered significant. Correlations between urine D-ribose levels and MMSE scores, as well as between urine formaldehyde levels and MMSE scores, were assessed using Spearman correlation methods. P value less than 0.05 (P ≤ 0.05) were considered significant. All statistical analyses were performed using origin 8.0 (Microcal, USA).
Results
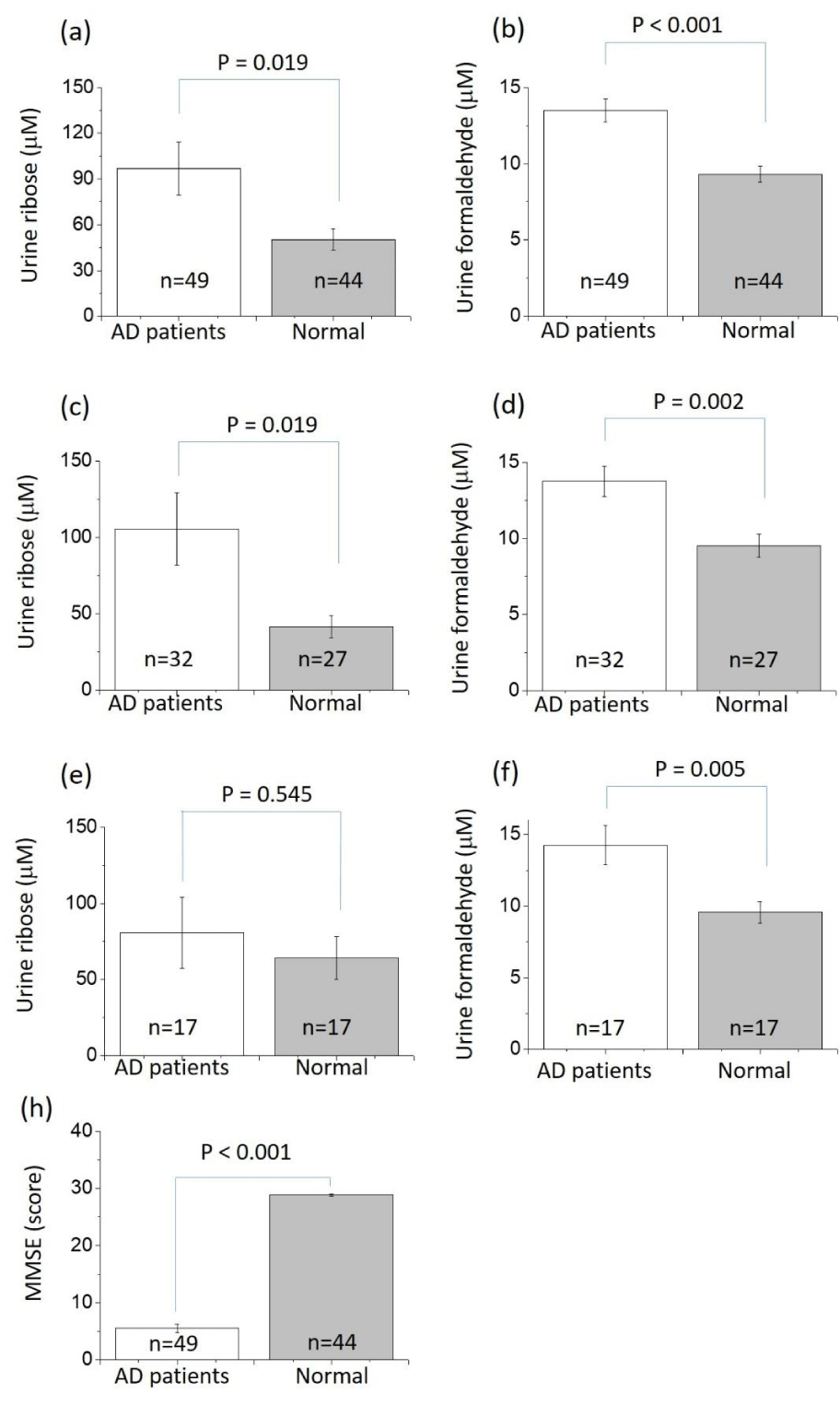
Nighty-three participants (aged 73.1 ± 9.6 year old) were enrolled, including 49 AD patients (32 female and 17 male) and 44 (27 female and 17 male) cognitively normal participants. Table 1 illustrates the demographic data and MMSE scores of the two groups of participants. MMSE scores, D-ribose levels and formaldehyde levels in urine of all the participants are shown in table 2. Comparison of urine D-ribose concentrations between AD patients and cognitively normal participants resulted significantly in difference (P < 0.05) as shown in figure 1a. Since the relation between endogenous formaldehyde and cognitive impairment has been found [28], we measured the formaldehyde concentration in urine as positive control. An extremely significant (P < 0.0001) difference of the formaldehyde concentration was observed between AD patients and cognitively normal participants (Figure 1b).
There are different variations in urine D-ribose levels in patients with AD of different genders. Significant (P < 0.05) difference was obtained between female patients and female controls (Figure 1c). However, the difference between male patients and male controls was not notable (P > 0.05, figure 1e) under the same determined conditions. Whereas, the formaldehyde level of patients with AD was significantly higher than cognitively normal participants of the two genders: Urine formaldehyde levels of female patients (P < 0.01, figure 1d) and male patients (P < 0.01, figure 1f). Differences in MMSE scores between AD patients and normal controls were remarkably examined under the same conditions (P < 0.0001, figure 1h).
In order to explore the relationship between D-ribose levels and cognitive abilities, we analyzed the Spearman correlation between the concentration of urine D-ribose (formaldehyde as reference) and MMSE scores. As shown in table 2, both concentrations of D-ribose (P < 0.01) and formaldehyde (P < 0.0001) were negatively correlated with MMSE scores in all the participants including the patients. For different genders, similar significantly negative correlations were observed in female participants for their urine D-ribose (P < 0.01) and formaldehyde (P < 0.001). Formaldehyde in male participants showed a significantly negative correlation with MMSE scores (P < 0.01), while D-ribose in male participant did not have the marked correlation (P > 0.05). Most interestingly, among cognitively normal participants, urine D-ribose showed a significantly negative correlation (P < 0.05) with MMSE scores, but not formaldehyde (P > 0.05). This appears that levels of urine D-ribose may be related to the cognitive change in the early stage of age-related cognitive impairment.
The average level of urine D-ribose of cognitively normal participants was 55.29 ± 7.08 µmol/ L in this study. As reported by Su and colleagues [11], the reference range of D-ribose in urine is 40-80 µmol/ L. The average level of AD patients D-ribose (96.91 ± 17.36 µmol/ L) was higher than this value, especially that of female AD patients (105.47 ± 23.61 µmol/ L) compared with the normal participants (41.58 ± 7.18 µmol/ L). The negative correlation between urine D-ribose and cognitive ability showed: The higher the D-ribose level is, the poorer cognitive ability the participant has. Among all AD patients, women were the major contributors to the elevation of urine D-ribose levels.
Discussion
Levels of D-ribose (or formaldehyde) in AD patients are negatively correlated with MMSE scores, suggesting the dysmetabolism of D-ribose may be one of the risks for AD. The effect of D-ribose on cognitive impairment may be resulted from these pathways:
• D-ribose can activate CaMKII, which catalyzes protein phosphorylation, leading to Tau hyperphosphorylation of rodents [29].
• Gavage of the D-ribose induces the formation of amyloid-β deposition in mouse cortex and hippocampus [21].
• Administration of D-ribose activates astrocytes and elevates levels of NFκB and TNFα in mouse brain and U251 and U87MG astrocyte cell lines in the irritation of inflammatory reaction [30].
• D-ribose is reactive in the glycation of haemoglobin [19] and serum proteins [18].
Glycation triggers the yield of reactive oxygen species (ROS), which is known to be harmful to cells [31]. Reduction of ROS promotes cell proliferation [32], which involved in age-related cognitive impairments [33]. Finally,
• D-ribose elevated levels of hepatic triglyceride through upregulation of Diacylglycerol O-Acyltransferase 1 (DGAT1) and DGAT2 [34], as triglycerides playing a role in cognitive dysfunction [35].
We have noticed that the correlation between the D-ribose levels and cognitive abilities is more likely to occur in female participants. D-ribose levels of female AD patients were notably higher than that of cognitively normal participants. However, significant differences were not observed in male AD patients. According to Schmidt and colleagues [36], there are sex differences in AD. First, the prevalence of AD is higher in women than in men [37]. Second, women have a broader spectrum of dementia related behavioral symptoms with a predominance of depression, while aggression is more frequent in men than in women [38]. Third, gender differences were also showed in expression of anti-oxidative enzymes and post-menopausal hormonal changes [39]. Finally, as described by Mathys and colleagues, an overrepresentation of female cells in AD-associated subpopulations, and substantially different transcriptional responses between sexes in multiple cell types, including oligodendrocytes [40]. Thus, the significant elevation of D-ribose levels also suggests the difference in the metabolism of D-ribose in female AD patients. Whether endogenous D-ribose could be selectively used for women with AD needs further clarifying.
There is more than one diagnostic criterion for AD, such as the National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria, the NINCDS-ADRDA criteria, the International Working Group (IWG) criteria, and so on. Their core points are consistent. There is lack of evidence of comparison of these criteria. We choose the NINCDS-ADRDA criteria which are most frequently used and best understood by Chinese clinicians according to the condition of the research settings to ensure the accuracy of the diagnosis. Due to the limitation of numbers of participants and difference of age distribution, further investigation with a larger population is needed to validate the diagnostic value of D-ribose in AD.
As reported by Takeuchi and co-workers, one of the patho-mechanisms of diabetes linked with AD is the non-enzymatic glycation [6]. Approximately 79.85% diabetic patients suffered from higher levels of serum D-ribose compared with the cognitively normal participants [31]. For diabetic AD patients, we suggested the intervention with their cognition as well as sugar metabolism [41]. It has been shown that suppression of Advanced Glycation End Products (AGEs) can prevent diabetes associated cognitive impairment in rat [42]. Massimo and colleagues found an association between diabetes and an approximately 65% reduction in risk of fast progression in AD [43]. It is possible that the better prognosis of the diabetic AD patients might be linked to the reduction of diabetes or AGEs [44]. This is to say, to regulate the metabolism of sugars (D-glucose and D-ribose) may be an adjuvant therapy for the diabetic AD patients.
In conclusion, D-ribose levels of AD patients were significantly elevated compared with those of age-matched cognitively normal control. AD patients suffered from high levels of urine D-ribose, especially in female AD patients. These data suggested that D-ribose can be used as a potential biomarker for age-related dementia, and that provides a clue to study the relation between D-ribose dysmetabolism and AD
Acknowledgement
This work was supported by grants from the Natural Scientific Foundation of China NSFC (31670805, 81573763), National Key Research and Development Program of China (2016YFC1305900; 2016YFC1306300), the Beijing Municipal Science and Technology Project (Z161100000217141; Z161100000216137), and the External Cooperation Program of BIC, CAS (20140909), and the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201834).
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001; 154: 635-641. https://bit.ly/2JHPtJe
- Sadowski M, Pankiewicz J, Scholtzova H, Li YS, Quartermain D, Duff K, et al. Links between the pathology of Alzheimer's disease and vascular dementia. Neurochem Res. 2004; 29:1257-1266. https://bit.ly/2Wx0sLk
- Lu JQ, Xia Q, Zhou Q. How to make insulin-producing pancreatic beta cells for diabetes treatment. Sci China Life Sci. 2017; 60: 239-248. https://bit.ly/2I0T1mp
- Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006; 9: 13-33. https://bit.ly/2YUEVKq
- Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer's disease: a population-based cohort study. Diabetologia. 2009; 52: 1031-1039. https://bit.ly/2HH419Q
- Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, et al. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000; 59: 1094-1105. https://bit.ly/2YUEb7X
- Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, et al. Advanced glycation end products in Alzheimer's disease and other neurodegenerative diseases. Am J Pathol. 1998; 153: 1149-1155. https://bit.ly/2K94EdM
- Nawale RB, Mourya VK, Bhise SB. Non-enzymatic glycation of proteins: a cause for complications in diabetes. Indian J Biochem Biophys. 2006; 43: 337-344. https://bit.ly/2wmNTnj
- Xu W, Mu YM, Zhao JJ, Zhu DL, Ji QH, Zhou ZG, et al. Efficacy and safety of metformin and sitagliptin based triple antihyperglycemic therapy (STRATEGY): a multicenter, randomized, controlled, non-inferiority clinical trial. Sci China Life Sci. 2017; 60: 225-238. https://bit.ly/2ECk9HA
- Wu Q, Wang XD, Gu Y, Zhang X, Qin Y, Chen H, et al. Screening and identification of human ZnT8-specific single-chain variable fragment (scFv) from type 1 diabetes phage display library. Sci China Life Sci. 2016; 59: 686-693. https://bit.ly/2VVRqTU
- Su T, Xin L, He YG, Wei Y, Song YX, Li WW, Wang XM, He RQ. The abnormally high level of Uric D-ribose for Type-2 diabetics. Progress in Biochemistry and Biophysics 2013; 40: 816-825.
- Keller PJ, Le Van Q, Kim SU, Bown DH, Chen HC, Kohnle A, et al. Biosynthesis of riboflavin: mechanism of formation of the ribitylamino linkage. Biochemistry. 1988; 27: 1117-1120. https://bit.ly/2I59B4A
- Seuffer R: [A new method for the determination of sugars in cerebrospinal fluid (author's transl)]. J Clin Chem Clin Biochem. 1977; 15: 663-668. https://bit.ly/2VQHfjn
- Chen L, Wei Y, Wang X, He R. D-Ribosylated Tau forms globular aggregates with high cytotoxicity. Cell Mol Life Sci. 2009; 66: 2559-2571. https://bit.ly/30K0KxL
- Chen L, Wei Y, Wang X, He R. Ribosylation rapidly induces alpha-synuclein to form highly cytotoxic molten globules of advanced glycation end products. PLoS One. 2010; 5: e9052. https://bit.ly/2VTOAPd
- Wei Y, Chen L, Chen J, Ge L, He RQ. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009; 10: 10. https://bit.ly/2W6AnU9
- Wei Y, Han CS, Zhou J, Liu Y, Chen L, He RQ. D-ribose in glycation and protein aggregation. Biochim Biophys Acta. 2012; 1820: 488-494. https://bit.ly/2Ma3u4k
- Chen Y, Yu L, Wang Y, Wei Y, Xu Y, He T, et al. D-ribose contributes to the glycation of serum protein. Biochimica et Biophysica acta Molecular basis of disease. 2019. https://bit.ly/2MeBsot
- Chen XX, Su T, Chen Y, He YG, Liu Y, Xu Y, et al. D-ribose as a contributor to glycated haemoglobin. E BioMedicine. 2017; 25: 143-153. https://bit.ly/2X9Ajzn
- Wang Y, Shi C, Chen Y, Yu L, Li Y, Wei Y, et al. Formaldehyde produced from D-ribose under neutral and alkaline conditions. Toxicology reports. 2019; 6: 298-304. https://bit.ly/2HVJjla
- Wu BB, Wei Y, Wang YJ, Su T, Zhou L, Liu Y, et al. Gavage of D-ribose induces A beta-like deposits, Tau hyperphosphorylation as well as memory loss and anxiety-like behavior in mice. Oncotarget. 2015; 6: 34128-34142. https://bit.ly/2VStNMb
- Liu KL, He YG, Yu LX, He RQ. Elevated formaldehyde in the cecum of APP/PS1 mouse. Microbiology China. 2017; 44: 1761-1766.
- Tong Z, Han C, Luo W, Wang X, Li H, Luo H, et al. Accumulated hippocampal formaldehyde induces age-dependent memory decline. Age. 2013; 35: 583-596. https://bit.ly/2HGkpHu
- He R. Formaldehyde and cognition. New York, NY: Springer. Dordrecht. 2017. https://bit.ly/2MfWliU
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982; 140: 566-572. https://bit.ly/2I5BzgL
- Wang ZYZ, MY Qu, GY Chen JX, Zhao J. The application of the Chinese version of mini mental state examination. Shanghai Psychiatry Medicine. 1989; 7: 108-111.
- Su T, Wei Y, He RQ. Assay of brain endogenous formaldehyde with 2, 4-Dinitrophenylhydrazine through UV-HPLC. Progress in Biochemistry and Biophysics. 2011; 38: 1171-1177.
- Tong Z, Zhang J, Luo W, Wang W, Li F, Li H, et al. Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiology of aging. 2011; 32: 31-41. https://bit.ly/2Qq0MpZ
- Wei Y, Han C, Wang Y, Wu B, Su T, Liu Y, et al. Ribosylation triggering Alzheimer's disease-like Tau hyperphosphorylation via activation of CaMKII. Aging Cell. 2015; 14: 754-763. https://bit.ly/2ECnhTQ
- Han C, Lu Y, Wei Y, Wu B, Liu Y, He R. D-ribosylation induces cognitive impairment through RAGE-dependent astrocytic inflammation. Cell death & disease. 2014; 5: e1117. https://bit.ly/2W9ypT7
- Fu JP, Mo WC, Liu Y, Bartlett PF, He RQ. Elimination of the geomagnetic field stimulates the proliferation of mouse neural progenitor and stem cells. Protein & cell. 2016; 7: 624-637. https://bit.ly/30MrBcF
- Zhang HT, Zhang ZJ, Mo WC, Hu PD, Ding HM, Liu Y, et al. Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase. Protein & cell. 2017; 8: 527-537.
- Bisht K, Sharma K, Tremblay ME. Chronic stress as a risk factor for Alzheimer's disease: roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol Stress. 2018; 9: 9-21. https://bit.ly/2Cb27uM
- Chen Y, Yu L, Wei Y, Long Y, Xu Y, He T, et al. D-ribose increases triglyceride via upregulation of DGAT in the liver. Science China Life sciences, 2019. https://bit.ly/2EANulO
- Sims RC, Madhere S, Gordon S, Clark E Jr, Abayomi KA, Callender CO, et al. Relationships among blood pressure, triglycerides and verbal learning in African Americans. Journal of the National Medical Association. 2008; 100: 1193-1198. https://bit.ly/2YOSue8
- Schmidt R, Assem-Hilger E, Benke T, Dal-Bianco P, Delazer M, Ladurner G, et al. Sex differences in Alzheimer disease. Neuropsychiatrie. 2008; 22: 1-15. https://bit.ly/2h0u5fI
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clinical epidemiology. 2014; 6: 37-48. https://bit.ly/2QBlNOF
- Bayles KA, Azuma T, Cruz RF, Tomoeda CK, Wood JA, Montgomery EB jr. Gender differences in language of Alzheimer disease patients revisited. Alzheimer disease and associated disorders. 1999; 13: 138-146. https://bit.ly/2X9rJRi
- Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. Journal of cellular and molecular medicine. 2017; 21: 1024-1032. https://bit.ly/2K9ZOgy
- Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, et al. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019. https://bit.ly/2HZQHMr
- He R. Formaldehyde and Cognition. Front Matter. In: Springer. Dordrecht. 2017. https://bit.ly/2I2jZu4
- Liu YW, Zhu X, Yang QQ, Lu Q, Wang JY, Li HP, et al. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology. 2013; 228: 585-594. https://bit.ly/2MfVi2s
- Musicco M, Palmer K, Salamone G, Lupo F, Perri R, Mosti S, et al. Predictors of progression of cognitive decline in Alzheimer's disease: the role of vascular and sociodemographic factors. J Neurol. 2009; 256: 1288-1295. https://bit.ly/2Kb9ozx
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet. 1998; 352: 837-853. https://bit.ly/2EyXWdq

Sign up for Article Alerts