In Hereditary Haemorrhagic Telangiectasia Pathological Nailfold Capillaroscopy is Associated with Pulmonary Arteriovenous Malformations?
Roberto Zarrabeitia1-3*, Elena Aurrecoechea4, Concepción Fariñas-Alvarez5, Ana Fontalba6, Jesús Zarauza7, Luisa Ojeda2,8, Jose A. Parra3,9
1Department of Internal Medicine, Hospital Sierrallana, Torrelavega, Spain
2Biomedical Research Centre Network for Rare Diseases (CIBERER), Spain
3Research Institute Valdecilla (IDIVAL), Santander, Spain
4Department of Rheumatology, Hospital Sierrallana, Spain
5Quality Unit, Marqués de Valdecilla University Hospital, Santander, Spain
6Department of Genetics, Marqués de Valdecilla University Hospital, Spain
7Department of Cardiology, Marqués de Valdecilla University Hospital, Spain
8Biological Research Center, Superior Council of Scientific Investigations (CSIC), Madrid, Spain
9Department of Radiology, Marqués de Valdecilla University Hospital, Spain
*Address for Correspondence: Roberto Zarrabeitia, Hospital Sierrallana, Barrio ganzo, s/n. 39300 Torrelavega, Cantabria, Spain, Tel: +34-942847400; Fax: +34-942847501; E-mail: roberto.zarrabeitia@scsalud.es
Submitted: 17 December 2016; Approved: 07 March 2017; Published: 10 March 2017
Citation this article: Zarrabeitia R, Aurrecoechea E, Fariñas-Alvarez C, Fontalba A, Zarauza J, et al. In Hereditary Haemorrhagic Telangiectasia Pathological Nailfold Capillaroscopy is Associated with Pulmonary Arteriovenous Malformations Adv J Vasc Med. 2017;2(1): 010-016.
Copyright: © 2017 Zarrabeitia R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Hereditary hemorrhagic telangiectasia; Nailfold capillaroscopy; Arteriovenous malformations; Liver transplantation; Vascular malformations
Download Fulltext PDF
Introduction and Aim: Nailfold capillaroscopy is used to assess vascularization in pathologies involving distal blood vessels. This study aims at describing nailfold capillaroscopy findings in a large cohort of Spanish Hereditary Hemorrhagic Telangiectasia (HHT) (Rendu Osler Weber) patients to correlate these findings with the presence of organ involvement.
Materials and Methods: During the period January 1, 2002 December 31, 2013 nailfold capillaroscopy with a digital microscope (100x) was performed on 195 HHT patients. Internal organ involvement was confirmed using Computed Tomography (CT) and altered Contrast Echocardiography (CEC) for Pulmonary Arteriovenous Malformations (MAVp), cranial magnetic resonance angiography for brain arteriovenous malformations, abdominal CT angiography for liver involvement and upper and lower endoscopy including video capsule endoscopy for gastrointestinal involvement.
Results: 107 women and 88 men were studied. Pathological findings were observed in 53.8% of patients. In HHT1 and older (> 50 years) this percentage reached 81.3% and 64.3% respectively; there were no significant differences considering gender. The most frequent observed pattern was the presence of mega capillaries (80%). The association between internal organ involvement and pathological capillaroscopy result was significant only in Pulmonary Arteriovenous Malformations (MAVp). The sensitivity of pathological capillaroscopy to detect MAVp was 85.4%.
Conclusion: Pathological capillaroscopy findings (especially mega-capillaries) are common especially in HHT1 patients and in elderly HHT patients. The association between pulmonary vascular malformations and pathological capillaroscopy result is highly significant. This observation could help to intensify lung screening and endovascular treatment in HHT patients.
Introduction
Hereditary Hemorrhagic Telangiectasia (HHT) better known as Rendu Osler Weber disease is an autosomal dominant genetic disease that is characterized by the development of abnormal small (telangiectasias) or large (arteriovenous malformations) vascularisation. These vascular abnormalities which may develop in any organ are responsible for the important morbidity and mortality of HHT. Penetrance of symptoms is variable, but generally increases with age, reaching 90% at the age of 45 [1]. The symptoms usually appear gradually and are influenced by factors such as age, sex, and genotype. Epistaxis, the most common clinical manifestation (up to 96% of patients) is usually the first symptom [2]. HHT is considered a rare disease since its average prevalence is estimated around 1 : 5000 - 1 : 8000 [3]. Thus it is still an under-diagnosed pathology [4], with significant morbidity and mortality [5] and with no definitive treatment except liver transplantation and interventional radiology procedures that could be considered curative in certain cases of liver and pulmonary vascular malformations.
The diagnosis is based on the Curaçao criteria: epistaxis, telangiectasia, dominant familial aggregation and involvement of internal organs. Presence of three or four of these criteria confirm the diagnosis of HHT [6,7]. Confirmation can nowadays be achieved by identifying the causative mutation through a molecular study. Three genes involved in the development of this disease have been identified so far. In HHT type 1 (first described) gene is the ENG one, located on chromosome 9 (9q33 - q34) that encodes for the protein endoglin [8-10]. HHT type 2 is caused by the mutation in the ACVRL1 gene, which encodes for the protein ALK1 (activin receptor-like kinase 1). This gene is located in the region 12q11 - q14 of chromosome 12 [11,12]. Regarding phenotype, HHT 1 patients show a higher prevalence of pulmonary and brain lesions while liver and gastrointestinal involvement are more frequent in HHT 2 [13]. Less than 2% of patients present a combined syndrome of HHT and Hereditary Juvenile Polyposis (JPHT) with phenotypical traits of HHT and a higher risk of malignant tumors in the gastrointestinal tract at an early age [15]. These cases are due to a third gene mutation, MADH4, located on chromosome 18 that encodes for Smad4 [14]. Recently, a new Syndrome: Capillary Malformations - Arteriovenous Malformations (SMCMA) has been described. This syndrome has a phenotype similar to HHT and it is caused by mutations in the gene that encodes for BMP9 (bone morphogenetic protein 9) [16]. All these proteins are involved in the cascade of extracellular-intracellular signaling of the TGF-β. This pathway is made up of more than 40 cytokines and it includes the three is of orms of TGF-β: TGF-β1, TGF-β2 and TGF-β3, five activins, 16 morphogenetic bone proteins, the anti-Müllerian hormone, and osteogenic, nodal and myostatin proteins [17-20]. All of them are involved in multiple biological processes such as the regulation of cell proliferation, differentiation, migration, and extracellular matrix formation, as well as the maintenance and repair of tissue homeostasis in adults. The cause of the pathogenicity of HHT in most cases is haploinsufficiency, with a deficit in the production of either endoglin, ALK1 or Smad 4 which are needed in minimal amounts by endothelial cells to operate properly.
Histologically, there are two types of vascular lesions in the disease
Telangiectasias: Small venular or arteriolar capillary vasodilations. These disappear with the diascopy and are located primarily in the skin and mucosal surface. In these lesions, a progressive arteriolization of circulation occurs which causes the venous-arterial capillary exchange area to disappear [21].
Arteriovenous Malformations (AVMs): These are abnormal direct communications between arteries and veins. A direct high flow communication can occur between the two vessels. A short circuit mediated by a nidus or a conglomeration of small intermediate vessels is also possible. The most affected organs are usually the lungs, liver, central nervous system, pancreas, and the gastrointestinal tract.
The capillaroscopy is a non invasive technique that, through a microscopic study of the nailfold capillaries, can be used to assess the state of the distal vascularization in certain systemic autoimmune diseases that also affect blood vessels [22] such as Systemic Sclerosis (SSc) [23], dermatomyositis, Mixed Connective Tissue Disease (MCTD) and lupus, and syndromes as Raynoud among others. There can be different patterns of capillary involvement depending on the case: an evolutive sclerodermiform pattern with enlarged capillaries and areas of microhaemorrhages and loss of capilarries in up to 80% patients with SSc [24]; up to 50% of patients with MCTD show an sclerodermiform pattern related with a more severe evolution than those with a tortuous pattern (15-20% of cases) [25]; tortuous pattern with subpapillary venous prominence and moderate capillary enlargement in patients with lupus (more frequent if associated with Raynaud syndrome or presence of anticardiolipin antibodies), and loss of capillaries, microhaemorrhages and megacapillaries in Raynaud’s phenomenon [26]. The TGF-β family of proteins is implicated in developing pathologies of the connective tissue as it regulates the genesis of the extracellular matrix in addition to angiogenesis. Excess of TGF-1 may contribute to the development of the disease. Drugs are being developed trying to reduce TGF-1 expression [27]. As in SSc, HHT is characterized by the presence of mucocutaneous telangiectasias and activation of the TGF-β signaling cascade in its pathogenesis. Therefore, in 2000, studies were carried out about the use of capillaroscopy in these patients [28]. As a result, megacapillary architectural disorders at the capillary level of the nail bed or in the back of the hands were observed in up to 83% of HHT patients [29,30]. These alterations may precede the onset of macroscopic mucocutaneous lesions and may help diagnosis at younger ages.
Patients and Methods
Patients
This work represents a descriptive, cross-sectional, observational study and included non selected adult and pediatric patients with diagnosis of HHT, either clinically or genetically and both symptomatic and asymptomatic attended at the HHT Unit in Hospital Sierrallana (Torrelavega, Cantabria, Spain) from January 1st 2002 to December 31st 2013. In this study, they were asked to undergo a capillaroscopy of the nailfold.
Digital capillaroscopy
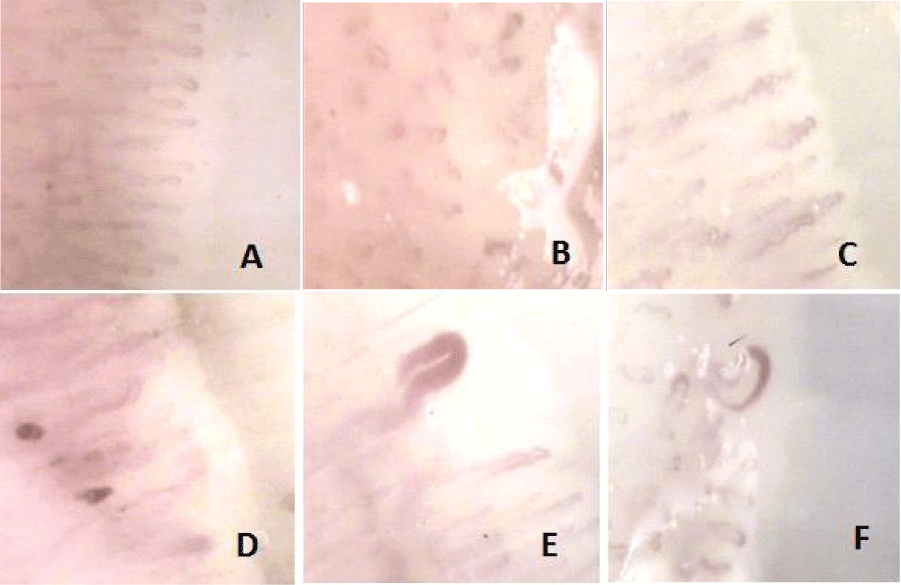
The capillaroscopy was always performed by the same observer, in this case by the author of the project in a room with temperature 20-23°C, after 15 minutes repose for patient. Among all parameters that characterize pathological capillaroscopy patterns only architecture was analyzed and evaluation was limited to the 4th and 3rd fingers of the right hand due to better epidermical transparence. Capillaroscopy was considered pathological when mega-capillaries (those enlarged 10 or more times compared with adjacent normal ones and so with a diameter over 50 microns) [31], microhaemorrhages, tortuosity (loops or patterns of capillaries “in fish banks”) were observed at the nail bed of the 4th and / or 3rd finger of the right hand.
Instrumental methods
A 100x digital microscope connected to a computer running the Avid Liquid© image viewing program was used to visualize nail bed of fingers.
Study protocol
The capillaroscopy findings were compared taking into account the variables of sex, age and HHT genetics. The simultaneous presence of mucocutaneous telangiectasias (and localization) and the involvement of internal organs were also considered in all patients included (symptomatic or asymptomatic): Lung involvement was determined when there were macroscopically visible MAVp in the thorax CT or considering the presence of a right-left shunting in the contrast echocardiogram performed with agitated saline solution and graded 0: no pass; 1: less than 20 bubbles; 2: 20-200 bubbles; 3: massive pass with left cavities preserved outline; 4: massive pass with left cavities blurred outline [32]. Cerebral involvement was defined as the presence of cerebral arteriovenous malformations in the cranial magnetic resonance angiography. Liver involvement was determined when telangiectasias or shunts were observed in an abdominal CT angiography. Finally, gastrointestinal involvement was determined when telangiectasias in the digestive tract were detected by gastroscopy, colonoscopy or a video capsule endoscopy.
Statistics
Data were summarized as mean and Standard Deviation (SD) for continuous variables, and frequency (%) for categorical variables. Statistical analysis was performed using two-tailed χ2 and Fisher´s exact test when necessary. A two-tailed p < 0.05 was considered statistically significant. Statistical data clean-up and analysis were performed with the SPSS 22.0 program (IBM Inc. Chicago IL, USA).
Ethics
All patients included in the study were asked to give their informed consent, in accordance with principles established in the Helsinki Declaration. The studies were approved by the Cantabrian Ethics and Clinical Research Committee.
Results
During the study period, 195 patients (107 women, mean age 45.25 ± 15, 52 and 88 men, mean age 46.18 ± 18.50) were evaluated. Of these, 105 had pathological findings in the capillaroscopy (53.8%). The main characteristics of the population are shown in table 1.
Within the group with pathological capillaroscopies, the proportion of women was slightly higher than that of men (54.2% versus 45.7%), without significant differences. A tendency towards a higher incidence of positive capillaroscopies in older patients was observed. This result was not statistically significant if the population was stratified into 5 groups (p = 0.14). However it was significant if the population was divided into either a group under 50 years old and another being 50 or over, with a higher prevalence of anomalies in the elderly (p = 0.011). There was a higher prevalence of capillaroscopic abnormalities in patients with HHT1 than in patients with HHT2 (p < 0.001).
Telangiectasias in additional areas were more frequent in patients with a pathological capillaroscopy. However, there was no significant difference, except in the case of labial lesions thus 61.9% of patients with lip telangiectasias showed a pathological capillaroscopy result (p = 0.017).
The most common abnormalities were mega-capillaries in 80 cases (41% of patients), loopings (3.6%), microhemorrhages (1.5%), “Fish bank” model (1.5%) and both megacapilaries with microhemorrhages in 4.6%. Images of different disorders observed are displayed in figure 1.
Comparing the results of the capillaroscopy and internal organ involvement (Table 2), the only statistically significant association was observed with lung involvement (both in the total population and considering gender) where 80.3% of patients presenting with MAVp in thorax CT had an abnormal capillaroscopy opposite to 31.2% with normal CT and pathological capillaroscopy (p < 0.0001). The relationship between a pathological lung-capillaroscopy condition remained statistically significant in the 16 to 65 year age group and in HHT1 patients (p = 0.016).
Depending on the degree of contrast shunt observed in the ECC, 46.7% of patients with grade 1-2 pass in ECC and 89.7% of those with grade 3-4 showed pathological findings in the capillaries. Thus a right to left shunt measured with contrast echocardiogram with a grade pass over 3-4 was significantly correlated with the finding of a pathological capillaroscopy result (p < 0.001).
In the same way the correlation between the results of a pathological capillaroscopy and a positive ECC was analyzed (to any grade pass) was also slightly significant (K = 0.32 with interval of confidence IC95 of 0.2 - 0.4).
The sensitivity of a pathological capillaroscopy for a mavp in the CT was 85.41% (IC95: 0.71 to 0.94), with a specificity of 54.54% (IC95: 0.46 to 0.62), a positive predictive value of 33.33% (IC95: 0.24 to 0.43) and a negative predictive value of 93.3% (IC95: 0.86 to 0.97).
Among children aged 0 to 15, the rate of a positive capillaroscopy was 50%. There were no clear differences in the genotypic distribution: of the 6 patients studied with a pathological capillaroscopy result, 4 were HHT1 and 2 were HHT2 cases. The affected group had only a slightly lower mean age (8.33 ± 3.01 versus 10 ± 3.41). Among HHT1 pediatric patients with an abnormal capillaroscopy, 3 had mega-capillaries and the other showed isolated microhemorrhages while in both HHT2 cases mega-capillaries were seen additionally with tortuous capillaries in loops in one of the patients.
Discussion
Although considering that up to 10% of healthy individuals can present with any isolate capillaroscopy disorders [25], patients with HHT show a high prevalence of capillaroscopy alterations, regardless of sex, especially in elderly patients and, strikingly, in patients with HHT1. Also a higher prevalence of pulmonary abnormalities was observed in HHT1 patients. This finding is consistent with the statistically significant association between a pathological capillaroscopy result and involvement of the lungs as well as the findings of a contrast echocardiography with a right-left shunt higher than grade 2. The sensitivity of a pathological capillaroscopy for detecting MAVp carriers in thoracic CT is fairly good (86%) with a good negative predictive value. Given the simplicity of the technique, and considering that the percentage of abnormalities could have even been higher if a complete capillaroscopy protocol evaluating 8 fingers had been performed, these results could mean that patients with a pathological capillaroscopy could be selected to perform a screening of pulmonary malformations in an even earlier stage than patients with a normal capillaroscopy. Due to the small number of patients with cerebral involvement, it could not be established if there was a significant relationship to pathological capillaroscopy, nor if there existed a relationship between liver and gastrointestinal involvement. This was because there was a higher rate of involvement in these locations in patients with HHT2. Most of the findings referred to mega-capillaries as described in the other series, corresponding to already completely dysfunctional and arteriolized vascular structures. However, micro hemorrhages and telangiectasias were also observed in fewer cases.
In a study carried out comparing patients with SSc and patients HHT (although low number) showed absence of differences in the alteration patterns with similar proportion of dilations and mega-capillaries in both groups and with no significant association with the presence of telangiectasias in additional locations [28]. Another study performed in the same year comparing a population of 54 patients with HHT with healthy patients, showed that there were mega-capillaries in 83% of cases with HHT. Meanwhile only 13% of patients were free of alterations with other 2 patients showing diffuse thickening in the area of the venous drainage. There was no involvement in the general population. Remarcable was that in 7 out of 9 patients with HHT, there were no visible mucocutaneous telangiectasias, so nailfold capillaroscopy lesions could precede those that are macroscopically visible in typical areas [29]. In 2005, Pasculli, et al. [30], presented a study with 88 patients on whom capillaroscopies of the nail bed and back of the hand were performed. This study showed a significantly higher prevalence of alterations in the latter location: there were 91% versus 7% mega-capillaries results in the same population. Finally in 2013, Riviere, et al [33], observed a pathological capillaroscopy result of 100% in 44 French patients. Of these, 70% had mutations, of which 61% were ENG and 39% were ALK1. Mega-capillaries in were observed in 43% of the patients, microscopic telangiectasia were observed in 70.5% of the patients and avascular zones were observed in 54% of the patients. Additionally Pulmonary involvement was significantly related to a pathological capillaroscopy result.
Conclusion
The nailfold capillaroscopy showed pathological results which were consistent especially in the presence of mega-capillaries in 53.85% of patients. The percentage of alterations increased with age, most significantly for those over 50. Genetically, the HHT1 population had higher rate of involvement (p < 0.0001). Considering the correlation with the existence of mucocutaneous telangiectasia at other levels, pathological capillaroscopy was associated only with the presence of labial telangiectasias. As for the relationship between capillaroscopic anomalies and internal organ involvement, there was a significant association between them in the case of lung disease considering thorax CT both in the total population and as defined by gender in the range of 16 to 65 years, as well as in HHT1 patients. The presence of an ECC with a grade pass of 3 - 4 was significantly associated with a pathological capillaroscopy result. The sensitivity of a capillaroscopy for the detection of MAVp in TAC was 85.41%.
The strength of this study is the sample size as it is the one of the largest series of HHT patients studied with the capillaroscopy technique, performed in this case by a single researcher in a specialized HHT unit with data of the screening protocol devoted to organ involvement identification.
The limits of the study are that there could be a bias regarding the origin of the population (Spanish, though from Mediterranean area), the low number of pediatric patients due to the characteristics of the hospital, the performance of a partial evaluation (4th - 5th fingers) and the absence of a control group of healthy subjects.
Key Messages
An abnormal nailfold capillaroscopy, mainly showing mega capillaries, is a common finding in HHT patients.
HHT 1 patients show a significant higher percentage of pathological findings.
A pathological nailfold capillaroscopy associated significantly with the presence of pulmonary arteriovenous malformations.
Acknowledgements
We acknowledge the Spanish HHT Patient´s Association for its inestimable collaboration.
Funding
This work was supported by funding from the Institute Carlos III (FIS PI 11/02426) also suitable to be cofunded with FEDER funds.
- Lenato GM, Guanti G. Hereditary haemorrhagic telangiectasia (HHT): genetic and molecular aspects. Curr Pharm Des. 2006; 12: 1173-1193. https://goo.gl/udXZZB
- AAssar OS, Friedman CM, White RI Jr. The natural history of epistaxis in hereditary haemorrhagic telangiectasia. Laryngoscope. 1991; 101: 977-980. https://goo.gl/7ZzHLz
- Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009; 17: 860-871. https://goo.gl/G4z2ip
- Latino GA, Brown D, Glazier RH, Weyman JT, Faughnan ME. Targeting under diagnosis in hereditary hemorrhagic telangiectasia: a model approach for rare diseases? Orphanet J rare Dis. 2014; 9: 115. https://goo.gl/FjgvGD
- Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999; 245: 31-39. https://goo.gl/O9N5tp
- Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Genet. 2000; 91: 66-67. https://goo.gl/qRtQX7
- Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, et al. International Guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. J Med Genet. 2011; 48: 73-87. https://goo.gl/lfOWm0
- Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990; 265: 8361-8364. https://goo.gl/JsWG4m
- McDonald MT, Papenberg KA, Ghosh S, Glatfelter AA, Biesecker BB, Helmbold EA, et al. A disease locus for hereditary haemorrhagic telangiectasia maps to chromosome 9q33-34. Nat Genet. 1994; 6: 197-204. https://goo.gl/nrTbzn
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, et al. Endoglin, a TGF-b binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994; 8: 345-351. https://goo.gl/hH8HyX
- Johnson DW, Berg JN, Gallione CJ, McAllister KA, Warner JP, Helmbold EA, et al. A second locus for hereditary hemorrhagic telangiectasia maps to chromosome 12. Genome Res. 1995; 5: 21-28. https://goo.gl/Kpf8tU
- Vincent P, Plauchu H, Hazan J, Faure S, Weissenbach J, Godet J. A third locus for hereditary hemorrhagic telangiectasia maps to chromosome 12q. Hum Mol Genet. 1995; 4: 945-949. https://goo.gl/w7vzcM
- Kjeldsen AD, Moller TR, Brusgaard K, vase P, Andersen PE. Clinical symptoms according to genotype amongst patients with hereditary haemorrhagic telangiectasia. J Intern med 2005; 258: 349-355. https://goo.gl/0SiBAq
- Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, et al. A combined syndrome of juvenile polyposis and hereditary hemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004; 363: 852-859. https://goo.gl/fYj263
- Wooder chak-Donahue WL, McDonald J, O'Fallon B, Upton PD, Li W, Roman BL, et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013; 93: 530-537. https://goo.gl/9U7dFz
- Canzonieri C, Centenara L, Ornati F, Pagella F, Matti E, Alvisi C, et al. Endoscopic evaluation of gastrointestinal tract in patients with hereditary hemorrhagic telangiectasia and correlation with their genotypes. Genet Med. 2014; 16: 3-10. https://goo.gl/hhSJNi
- Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003; 98: 257-265. https://goo.gl/jbvsph
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Bio chem. 2010; 147: 35-51. https://goo.gl/OQanqW
- Di Clemente N, Belville C. Anti-Mullerian hormone receptor defect. Best Pract Res Clin Endocrinol Metab. 2006; 20: 599-610. https://goo.gl/oaTCdL
- Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-β). Growth Factors. 1993; 8: 1-9. https://goo.gl/hmh63O
- Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol. 1990; 95: 422-427. https://goo.gl/SbxuJj
- Cutolo M, Smith V. State of the art on nail fold capillarosocopy: a reliable diagnostic tool and putative biomarker in rheumatology? Rheumatology (Oxford). 2013; 52: 1933-1940. https://goo.gl/hnyNaZ
- Ennis H, Moore T, Murray A, Vail A, Herrick AL. Further confirmation that digital ulcers are associated with the severity of abnormality on nail fold capillaroscopy in patients with systemic sclerosis. Rheumathology (Oxford). 2014; 53: 376-377. https://goo.gl/MvgFmd
- Cutolo M, Pizzorni C, Tuccio M, Burroni A, Craviotto C, Basso M, et al. Nail fold videocapillaroscopic patterns and serum antibodies in systemic sclerosis. Rheumatology (Oxford). 2004; 43: 719-726. https://goo.gl/f1JNpR
- García-Patos V, Fonollosa V. Utility of the nail fold capillaroscopy. Jano. 2002; 60: 64-68.
- Anamaria da SF, Mario LCP, Mônica Ribeiro AV, Luci BF, Augusto de AF. Capillary findings in lupus erythematosus. An Bras Dermatol. 2006; 81: 527-532. https://goo.gl/fCmmb5
- Gil-Guerrero L, Dotor J, Huibregtse IL, Casares N, López-Vázquez AB, Rudilla F, et al. In vitro and in vivo down-regulation of regulatory T cell activity with a peptide inhibitor of TGF-beta 1. J Immunol. 2008; 181: 126-135. https://goo.gl/hl5k2h
- Mould TL, Roberts-Thomson PJ. Pathogenesis of telangiectasia in scleroderma. Asian Pac J Allergy Immunol. 2000; 18: 195-200. https://goo.gl/PkJqmr
- Mager JJ, Westermann CJ. Value of capillary microscopy in the diagnosis of hereditary hemorrhagic telangiectasia. Arch Dermatol. 2000; 136:732-734. https://goo.gl/Uneb8t
- Pasculli G, Quaranta D, Lenato GM, Suppressa P, Lastella P, Guanti G, et al. Capillaroscopy of the dorsal skin of the hands in hereditary hemorrhagic telangiectasia. QJM. 2005; 10: 757-763. https://goo.gl/rmVbrD
- Cutolo M. Atlas of capillaroscopy in rheumatic diseases. Milan: Elsevier. 2010; P: 5-33. https://goo.gl/a3s7nt
- Parra JA, Bueno J, Zarauza J, Fariñas-Alvarez C, Cuesta JM, Ortiz P, et al. Graded contrast echocardiography in pulmonary arteriovenous malformations. Eur RespirJ. 2010; 35: 1279-1285. https://goo.gl/lZl9d3
- Riviére S, Marnas E, Van Kien AK, Laroche JP, Lorcerie B, Quere I. Capillary microscopy in hereditary hemorrhagic telangiectasia: a prospective study of 44 patients. Hematol Meet Rep. 2013; 5: 49-50.

Sign up for Article Alerts