Extraction and Partial Purification of Peroxidase Enzyme from Plant Sources for Antibody Labeling
Gutema Dinkisa Idesa1* and Belayneh Getachew2
1Mekelle university, College of veterinary medicine, Ethiopia
2National veterinary institute(NVI), Bishoftu, Ethiopia
*Address for Correspondence: Gutema Dinkisa Idesa, Veterinary Medicine, Mekelle University, in Partial Fulfillment for the Attainment of Degree of Doctor of Veterinary Medicine, Tel: +251-920-910-284; E-mail: gutemadin@gmail.com
Submitted: 27 April 2018; Approved: 17 May 2018; Published: 18 May 2018
Citation this article: Idesa GD, Getachew B. Extraction and Partial Purification of Peroxidase Enzyme from Plant Sources for Antibody Labeling. Int J Vet Sci Technol. 2018;2(1): 006-012.
Copyright: © 2018 Idesa GD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Enzyme; Extraction; Ipomoea batatas; Peroxidase; Solanum tuberosum
Download Fulltext PDF
This study was conducted from November 2016 to May 2017 in Mekelle to extract and partially purify peroxidase enzyme from Ipomoea batatas, Solanum tuberosum, Daucus carota, Daucus carota and Schinus molle for use in labeling of antibody. Partial purification was done with ammonium sulphate precipitation along with dialysis tubing and the activity of extracted plants enzyme was determined spectrophotometrically. An increase in peroxidase enzyme activity was observed from all plants after partial purification. Among the tested plants, Ipomoea batatas showed promising enzyme activity on crude extract with specific peroxidase activity of 479 u/mg. Solanum tuberosum resulted in highest peroxidase activity after partial purification with specific peroxidase activity of 2567.81 u/mg. Moderate enzyme activity was observed from both crude and partially purified extracts of Daucus carota with 353.42 u/mg and 1852.27 u/mg, respectively. The extracts of both Daucus carota and Schinus molle showed slight peroxidase activity before and after partial purification. The results suggest that Solanum tuberosum and Ipomoea batatas could be a rich source of peroxidase enzyme. Further studies on natural peroxidase rich plants with sophisticated techniques and a trial of conjugating this plant peroxidase to antibodies as labels for ELISAs are recommended.
Abbreviations
BSA: Bovine Serum Albumin; ELISA: Enzyme Linked Immunosorbent Assay; HRP: Horseradish Peroxidase; OPD: Ortho Phenylenediamine Dihydrochloride; PBS: Phosphate Buffered Saline; POD: Peroxidase; RPM: Rotations Per Minute.
Introduction
Enzyme immunoassays have important practical applications in veterinary diagnostic laboratories for the detection of infectious agents that cause disease in animals. Such assays generally rely on coupling of marker enzymes such as horseradish peroxidase with antigens or antibodies which later brings color change due to the functional alteration of an enzyme or antibody for determination of the presence or amount of antigen in samples [1]. Direct conjugation of enzymes to antibodies has greatly simplified the development and performance of many different types of immunoassays. An enzyme-labeled secondary antibody is used to detect bound antibody or antigen that leads to a color change upon addition of enzyme substrate and the intensity of the color that develops, is therefore proportional to the amount of enzyme-linked antiglobulin that is bound, which in turn is proportional to the amount of antibody present in serum or antigen in sample under test [2]. The most popular enzymes that have been used in Enzyme-linked immunosorbent assay kits include alkaline phosphatase, horseradish peroxidase and β-galactosidase. Among which peroxidase is widely used to prepare antibody-enzyme or anti-antibody-enzyme conjugates [3].
Peroxidase, a biotechnologically important enzyme is a ubiquitous enzyme which belongs to the oxidoreductase class of enzyme and generally catalyzes a reaction between hydrogen peroxide as electron acceptor and many kinds of substrates by means of oxygen liberation [4]. It is a heat stable, intracellular enzyme widely distributed in nature and is found in plant kingdom, micro-organisms and animals where it catalyze the reduction of hydrogen peroxide to water, rendering it harmless [5].
Plants are the rich sources of peroxidase and primarily found in roots and sprouts of higher plants. The Plant Peroxidase (POD) superfamily comprises heme-containing glycoproteins that differ in their structure and catalytic properties. The enzyme is present naturally in plants like potato tuber, horse radish, beet, soybean, tomato, banana, papaya, carrot, turnip, wheat, dates, beats and strawberry [6-7].
Enzymes used as labels for Enzyme-Linked Immunosorbent Assays (ELISAs) should meet requirements such as stability at typical assay temperatures (4°C, 25°C, and 37°C), greater than six months shelf life when stored at 4°C, capable of being conjugated to an antigen or antibody, inexpensive, easily measurable activity, high substrate turnover number, unaffected by biological components of the assay, high purity, high conversion rate, favorable specificity, rich resources, and remaining active component and catalytic capacity after becoming conjugate. Horseradish Peroxidase (HRP) conjugates are ideal for use in immunoassays such as ELISA and other applications where high specificity and low background noise are important, such as when sample volume is limited or when the target molecule is present at low levels. Such conjugate have a very high turnover rate, rapid availability, easy of conjugation, better sensitivity and stability with short reaction times [8].
Peroxidase enzyme is become important industrially and medically gaining great economic importance through its uses in the number of diagnostic tools and in the analysis of numerous assays because of its high affinity toward the material basis, easy of detection effectiveness configures, outputs of color, does not need a process to measure the steps separating from substrates, high persistence during storage, low cost preparation and purification [9]. In the biological field, it is having wide range of applications as diagnostic kits for enzyme immunoassays and as an important component of ELISA [10]. It is preferred for preparing Enzyme conjugated antibodies due to its high specificity towards certain substances, and hence widely used in ELISA for labeling antibodies or antigens in the immune reactions by attaching these enzymes on solid surfaces and other sensitive analytical techniques [11].
The manufacture of enzyme immunoassay kits and medical diagnosis kits uses POD most frequently. In fact, more than 90% of the immune enzymatic kits are prepared using peroxidase as label enzyme to generate immune conjugates. It is used in the enzymatic determination of glucose plasma concentration [12], and to quantify a number of compounds using ELISA tests, such as therapeutic drugs [13], viruses [14].
Peroxidase has been isolated and characterized from a large number of plant sources like fruits, leaves, tubers and grains. However, its availability with higher stability and different specificity would improve immune enzymatic analytical kits and promote the development of new analytical methods and potential industrial processes.
The plants used in this study were bulbs of Ipomoea batatas (sweet potato), Solanum tuberosum (potato), fruit of Daucus carota (tomato), root of Daucus carota (carrot) and leaf of Schinus molle (Peruvian pepper). All these plants have been widely used in the communities for different purpose since early time and have worldwide distribution. Extraction (as the term is pharmaceutically used) is the separation of active portions of plant (and animal) tissues using selective solvents through standard procedures. Such extraction techniques separate the soluble plant metabolites and leave behind the insoluble cellular marc [15].
Purification means sequences of chain from separation methods that could separate specific protein from other proteins and compounds exist in enzyme extract. In areas where there is lack of infrastructures, Partial purification also increases specific activity to enzyme through certain steps that would lead to purified and homogenized enzyme [16].
Although peroxidases are ubiquitous in the plant kingdom, at present the major source of commercially available peroxidase is the roots of horseradish plant from which many countries are producing. A major limitation for the wide spread use of POD is the current high cost of production of the enzyme. The enzyme cost can be reduced either by reducing the production cost and/or by reducing its recovery and purification cost [17].
Regardless of its wide use in animal disease diagnosis and other areas, nevertheless, no remarkable work on this enzyme has so far been done in Ethiopia to produce it commercially. Horseradish plant is not available in our country unlike most eastern Asian and European countries and the peroxidase content of locally available plants was not yet examined for any purpose. The commercial peroxidase in a conjugated form with secondary antibody along with ELISA kits are therefore, being imported with very high price to use by fewer research institutions and colleges for enzyme immune assays. Thus there is a need to commercialize and standardize these enzymes and kits depending upon our own sources and technology to reduce burden on our economy.
The indigenous natural plant resources could provide fresh and cheaper raw material for large scale peroxidase extraction and purification. Local plants, grown in Ethiopia may be explored for Peroxidase yield and could be an alternative commercial source of high activity peroxidase enzyme.
Therefore, the objectives of this research were:
● To extract and partially purify peroxidase enzyme from plant sources as alternative to commercially available HRP for antibody labeling
● To identify plants rich in peroxidase by determining their peroxidase enzyme activity.
Materials and Methods
Study area
This study was carried out in Mekelle from November 2016 to May 2017. Mekelle is the capital city of Tigray regional state located in the northern extremes of Ethiopia extending from 33° 251 to 390o 381 north latitudes and from 36° 271 to 40° 181 east longitudes at an average altitude of 2000 to 2200 meters above sea level. The mean annual rain fall ranges from 11.3 mm to 39.1 mm and the temperature varies from 12°C (in November and December) to 27°C (in January and March). Mekelle enjoys humid and hot climate and 783 kilometres far from Addis Ababa, capital city of Ethiopia [18].
Study design
This investigation employed a laboratory based enzyme extraction and partial purification with ammonium sulphate precipitation and dialysis tubing, and also the determination of peroxidase activity spectrophotometrically using Ortho-phenylenediamine Dihydrochloride as indicator at 492 nanometer [19].
Plant collection and preparation of enzyme extract
The plants used for this study were intentionally selected and purchased from local market after their screening test showed possibility of Peroxidase content. After the preliminary operations like washing, peeling and slicing, the plants were crashed with pestle and mortar to obtain a homogenous sample and often to improve the kinetics of analytic extraction and also to increase the contact of sample surface with the solvent system.
Crude enzyme extracts were prepared by grinding 100 g of each plant in 400 ml of distilled water separately. The crushed extracts were homogenized thoroughly by blending for 15 min and were centrifuged at 6000 Rotations Per Min (RPM) for 15 min. The supernatant was filtered through sieve and the sediment was discarded. To selectively inactivate the contaminating traces of the catalase moieties, crude enzyme extract was heated at 65°C for 3 min in a water bath and cooled promptly by placing it in ice bucket for 30 min [20]. After thermal inactivation, the enzyme activity, protein content and specific enzyme activity of final extracts were determined and they were preserved at -20°C until partial purification was done.
Partial purification and dialysis of peroxidase
The partial purification step was made at room temperature with buffer solution of pH 7.4. The enzyme extract was subjected to ammonium sulfate precipitation by the method [21]. Ammonium Sulfate (NH4SO4)2 was added to the crude enzyme extract until it was 80% saturated by constant stirring with magnetic stirrer to ensure complete solubility. Then, it was centrifuged at 5000 RPM for 30 min. After centrifugation, the supernatant was discarded and the sediments were dissolved in small amount of phosphate buffered saline in which the enzyme was originally extracted. The solution was kept in a dialysis bag after sealing securely, and dialyzed against Phosphate Buffered Saline (1xPBS) for 24 hr by the use of dialysis tubing having dialysis membrane of 25 kilo Dalton (narrow enough to trap POD which is 44 kilo Dalton). The collected fractions were analyzed for enzyme activity and total protein content. The specific enzyme activity was calculated and, the values were compared with that of crude extract.
Protein estimation of enzyme extracts
Total protein contents of the extracts were measured by biuret method [22] using Bovine serum albumin as standard for calibration. Different concentrations of Bovine Serum Albumin (BSA) (0, 0.4, 0.8, 1.2, 1.6 and 2 mg/ml) and plant extracts were poured into test tubes and their reading was taken after addition of biuret reagent. All tubes were incubated at room temperature for 20 min and reading was taken at 540 nm. Protein concentration of the extracts was estimated from their absorbance reading by drawing the points with protein concentration of BSA as x’ values and their average absorbance as y’ values on a graph paper.
Peroxidase enzyme assay
Serial dilutions of enzyme extracts were examined for peroxidase activity assay. The assay was done using Ortho-Phenylenediamine Dihydrochloride (OPD) as Substrate at room temperature. OPD, in the presence of peroxidase enzyme, reacts with hydrogen peroxide in a one to one stoichiometry to produce 2, 3-diaminophenazine, yellow orange compound which has absorption maxima of 492 nanometer. The formation of such color along with its intensity uses to indicate the availability of target enzyme with its concentration. The change in absorbance at 492 nm due to the oxidation of OPD in the presence of hydrogen peroxide and enzyme extract at room temperature was monitored using Multiskan Spectrophotometer.
2H2O2 Plant Peroxidase 2H2O + O2
OPD + O2 Oxidized OPD (colored)
The standard assay solution contained 0.4 mg/ml of OPD, 0.01 ml of 3 % hydrogen peroxide, 0.09 ml of distilled water and 0.1 ml of enzyme extract in total of 0.2 ml. Control assays in which the enzyme extracts or substrates were replaced by buffer were performed. The change in absorbance was recorded for every 30 sec for 5 min during the reaction. One unit of peroxidase activity (u) represents the amount of enzyme catalyzing the oxidation of 1 µmol of OPD in 1 min under the assay conditions [23]. The total enzyme activity of Plant was calculated according to [24] as follows:
Units/ml = change in absorbance per min x dilution factor x 1000
ml of enzyme used in the assay
Specific peroxidase activity is the ratio of enzyme activity (u/ml) to protein concentration (mg/ml) that is expressed in u/mg of protein and it was calculated [25].
Data Analysis
Data on absorbance value for each crude and partially purified enzyme extracts of plants was stored in excel spreadsheet. Simple descriptive statistics were used to determine the mean of different results. All reading assays were performed using a Multi scan spectrophotometry coupled to a computer and printer. The values reported are the mean of at least three independent determinations. All these were done using statistical package for the social sciences software version 20.
Results
Out of the five plants used in this study, maximum yield of peroxidase enzyme in crude extract was obtained from Ipomoea batatas which was 479 u/mg followed by Daucus carota, Solanum tuberosum, Daucus carota and Schinus molle with yields of 355.42 u/mg, 278.05 u/mg, 192.91 u/mg and 4.89 u/mg respectively. The least yield of enzyme activity was found from crude extract of Schinus molle (Table 1).
It was observed that after partial purification, the enzyme activity of Solanum tuberosum and Daucus carota was markedly increased as compared to crude enzyme extract. Extracts of other plants were also increased in activity after ammonium sulfate precipitation. The protein contents of each enzyme extracts was diminished to great level after partial purification by ammonium sulfate precipitation which indicated that unnecessary proteins were salted out. Marked reduction of protein was observed from extract of Schinus molle (Table 2).
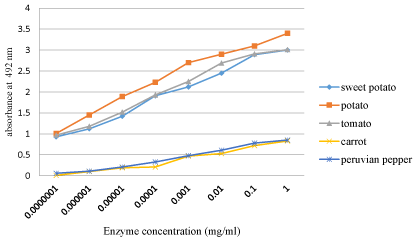
Serial dilutions of each enzyme extract were done, where potato peroxidase showed a detectable color change with unaided eye upto 10-7 dilution and an optical density of 1.01 absorbance. Sweet potato and tomato peroxidase were also resulted in 0.93 and 0.97 absorbance at similar dilutions, respectively (Figure 1).
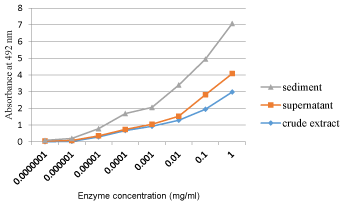
Sediment and supernatant of partially purified enzyme extracts were both showed peroxidase activity with highest values were observed from sediments of all plant extracts. However, the supernatant showed lowest enzyme contents even below crude extracts of plants (Figure 2).
Discussion
Crude peroxidase enzyme was extracted from potato, sweet potato, tomato, carrot and Peruvian pepper plants using distilled water as solvent at room temperature by blending the plants for 15 min with short intermissions and centrifuged at 6000 RPM for 15 min to remove particulate matter and any intact nuclei from solution. The intensity of the characteristic yellow orange color produced during reaction expressed the amount of enzyme and the magnitude of enzyme reaction in the crude extract. Enzyme activity determination was based upon the rate of utilization of substrate or formation of product per unit time under controlled conditions. The enzyme activity in terms of chromogenic reaction development was quantified spectrophotometrically, with the increase in reaction period, a constant trend of increase in the absorbance values in POD in all sources was observed. To purify the desired enzyme, extract was subjected to 80% saturation with (NH4)2SO4. It is the most commonly used reagent for salting out of the protein because of its high solubility permits the achievement of solution with high ionic strength [26].
The mean increase in absorbance of crude of potato, sweet potato, tomato, carrot and Peruvian pepper was 1.11, 0.74, 1.01, 0.1, and 0.25, respectively after partial purification step. Similarly activity of POD at various dilutions and at different time interval varied significantly, which approved that all the plant sources differed from one another significantly. This remarkable difference between the optical density values were attributed to the variation in the POD activity in plants in relation with anatomical locations, age of tissue/plant, physiological activity and state of being fresh or stocked [27].
In this study, all extracts of the tested plants showed promising availability of peroxidase enzyme activity in them. This implies that the test plants could be potentially being a source of commercial peroxidase enzyme in the future. Another important point was the major difference in enzyme activity from sediment and supernatant of extracts. The result of this study is in agreement with [4] were their investigation resulted in major enzyme activity obtained from sediments after centrifugation. Free Peroxidase settles on addition of salts that compete for water molecules in bond formation. This proved that precipitation by salt for protein purification is good to isolate them from a diluted solution of numerous mixtures. The minimum amount of enzymes that were found in supernatant was probably due to ineffective centrifugation being used.
Previous studies show the influence of the solvents in the extraction of enzymes from plant materials [28]. Reported that, organic solvents are better in extracting peroxidase from natural plant sources. An interesting point in this study was the effect of the substrates (hydrogen peroxide and OPD) concentration on peroxidase activity showed that the activity of peroxidase increased with corresponding increase in substrates concentration with maximum saturation point of about 3 % for hydrogen peroxide and 0.4 mg/ml for OPD, indicating that the active sites are saturated with the substrates.
The pH of the solvents, temperature, type and concentration of substrates can also have influence on the peroxidase activity of plants [29]. Reported that the purification of peroxidase from Gongronema latifolium using Guiacol as substrate yielded a higher purification fold when compared with OPD at optimum temperature of 30oC and pH of 7 respectively. On this study 80 % saturation of (NH4)2SO4 was found best where all extracts showed good enzymatic activity after purification [21]. Standardized the saturation values of (NH4)2SO4 as 50-85%, while [30] purified peroxidase from different seeds like horseradish with 35-90% precipitation. The finding of [7] was 1.21 fold purification for soybean peroxidase using the same 85% saturation values. This difference may be due to grade of salt used for purification or the concentration of salt and duration of saturation for purification.
In general the concentration of OPD (0.4 mg/ml) used in this study is equal the values used in other enzyme assays. The peroxidase activity exhibited at 0.25 g/ml concentration of the plant extracts was found comparable to those of sophisticated studies. PBS was also used in place of distilled water and showed no differences on peroxidase activity. The mixtures of distilled water and enzyme extract were used as negative control in absence of substrate and there was no peroxidase activity and the results obtained from this study were purely related to the peroxidase enzyme content of each plant.
The change in wave length reading of spectrophotometer was tested on all enzyme extracts of plant to get maximum yield, were 492 nm resulted in highest reading. This is not in agreement with the finding of [31] who got maximum yield by reading at 460 nm and [4] at the wavelength of 470 nm. This indicates that the environmental conditions might have an influence on the nature and concentration of plant peroxidase.
The extracts of all plant at concentration of 0.25 g/ml did show peroxidase activity before and after partial purification step. The maximum enzyme activity was obtained from Ipomoea batatas upon crude extract evaluation of 479 u/mg of proteins which was followed by Daucus carota with 355.42 u/mg. This result is in agreement with POD extraction from orange waste which resulted in 734.1 u/mg of protein [32], but differs from result of [33] (14 u/mg). Plant extraction at 0.1 mg/ml did show minimum enzyme activity with minimum optical density. After partial purification with 80 % saturation of ammonium sulfate, Solanum tuberosum showed high peroxidase enzyme activity with 2567.81 u/mg of protein that showed that the dialysis step removed numerous unwanted proteins and improved a way towards clear enzyme product. The results showed this newly extracted peroxidase was as active as the most commonly using enzyme in antibody conjugation, Horseradish Peroxidase Enzyme (HRP), towards its most common substrate, Ortho-phenylenediamine Dihydrochloride.
Conclusion and Recommendations
Plant enzymes such as peroxidase conjugated with animal antibody are widely used in veterinary as a component of ELISA kits and in other diagnostic tools. Extraction and partial purification of plant peroxidase enzyme was done from cost effective sources with simple laboratory technique and inexpensive chemicals like ammonium sulfate where major laboratory equipment are lacking. In this study Solanum tuberosum has showed promising peroxidase enzyme activity after partially purified by ammonium sulfate and this could be a rich source of peroxidases for enzyme immunoassays. Ipomoea batatas and Daucus carota have also showed a moderate peroxidase enzyme activity. Potato and sweet potato peroxidase could be suggested as a replacement candidate for horseradish peroxidse, especially in Ethiopia where these plants could be obtained cheap and fairly fresh. However, Daucus carota and Schinus molle showed only slight enzyme availability.
Therefore, from the finding of this study the following recommendations are forwarded:
● Extraction and purification of peroxidase from other cheap and natural sources should be attempted for future enzyme immunoassay activities
● Further studies, especially on Solanum tuberosum, should be made in order to get pure and homogeny peroxidase
● In addition, conjugation of these plants peroxidase with antibody should be done to determine their potential in labeling of ELISA
Annexes
Preparation of phosphate buffered saline (1X PBS)
PBS is a buffer solution commonly used in biological research. It is a water-based salt solution containing sodium hydrogen phosphate, sodium chloride and, in some formulations, potassium chloride and potassium dihydrogen phosphate. Some formulations of PBS do not contain potassium, while others may contain calcium or magnesium. For this study 1X PBS was prepared as mentioned in the table below:
● First, salts were added to 800 mL of distilled water and dissolved well. Finally pH was adjusted to 7.4 with Hydrochloric acid and distilled water was added to bring to a final volume of 1 liter.
Biuret reagent preparations (per liter final volume)
Biuret reagent was prepared as follows for qualitative protein determination of plant extract in order to get specific enzyme activity:
• 9 grams of sodium potassium tartarate
• 3 grams of copper sulfate x5H2O2
• 5 grams of potassium iodide
• All dissolved in order in 400 ml 0f 0.2M sodium hydroxide before bringing to final volume (1L)
3. Preparation of working substrate for peroxidase assay (0.4 mg/ml OPD and 3 % H2O2)
o In the first test tube, 0.9 ml of distilled water, 0.1 ml of 30 % H2O2 and 40 mg of OPD were added
o In the second test tube, 0.81 ml of dH2O2, 0.09 ml of 30 % H2O2,and 0.1 ml of solution from test tube 1 were added which resulted in 0.4 mg/ml OPD and 3 % H2O2
Acknowledgements
Praise to the Almighty God, king of the Universe he who granted me the opportunity to live and for owing me strength and patience throughout my life.
My special thanks and gratefulness goes to my advisor Dr. Belayneh Getachew (DVM, MSc, PhD) for his invaluable guidance, genuine encouragement, intellectual advice and provision of necessary materials during working time.
Thanks and gratitude to Ministry of Science and Technology of Ethiopia for their financial support in providing chemicals and materials essential for the study.
I would also like to express my deep sense gratitude and sincere thanks to my family for their overwhelming inspiration and support.
- Schmitt B. and Henderson L. Diagnostic tools for animal diseases. Rev Sci Tech. 2005; 24: 243-250. https://goo.gl/vUiwcb
- Tizard IR. Immunodiagnostic techniques. An introduction to Veterinary Immunology. 7th ed. 2004; 184-189. https://goo.gl/LTiuJn
- Kemeny DM, Challacombe SJ. ELISA and other solid phase Immunoassays. John Wiley and Sons. 1989; 1-16. https://goo.gl/Kgj2NW
- Zia MA, Kousar M, Ahmed I, Iqbal HMN, Abbas RZ. Comparative study of peroxidase purification from apple and orange seeds. African Journal of Biotechnology.2011; 10: 6300-6303. https://goo.gl/9hgn6C
- Burnette F. Peroxidase and its relationship to food flavor and quality: A review. Journal of food Science. 1977; 42: 1-6. https://goo.gl/eSGgQF
- Reed G. Oxidoreductase: Enzymes in food processing. Academic Press, USA P. 1975; 216.
- Ambreen SK, Rehman M, Zia A, Habib F. Kinetic studies and partial purification of peroxidase in soybean. Pakistan Journal of Agricultural sciences. 2000; 37: 119-122.
- Zia MA, Yaqub K, Rehman R, Mahmood T. Production of rabbit antibuffalo antibodies horseradish peroxidase conjugate and standardization of ELISA for Pasteurella multocida antibodies. Pakistan Journal of Biological Science. 2000; 3: 1716-1718. https://goo.gl/LKgfyU
- Tijseen P. Properties and preparation of enzymes used in enzyme immunoassays. In Laboratory techniques in biochemistry and molecular biology, practice and theory of enzyme immunoassays. Elsevier, Amsterdam, New York. 1985. 15: 173-219. https://goo.gl/WWdJhg
- Deepa S, Arumughan C. Purification and characterization of soluble peroxidase from oil palm (Elaeisguinensis jacq) leaf. Phytochemistry. 2002; 61: 503-511. https://goo.gl/4fRBF5
- Kemeny DM, Challacombe SJ. ELISA and other solid phase Immunoassays. John Wiley and Sons. 1989; 1-16. https://goo.gl/Kgj2NW
- Krell HW. Peroxidase: an important enzyme for diagnostic test kits. Biochemical, molecular and physiological aspects of plant peroxidases. University of Lublin, Poland, and University of Geneva. 1991; 1: 469-478.
- Ternant D, Mulleman D, Degene D, Willot S, Gullaumin JM, Watier H, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit.2006; 28: 169-174. https://goo.gl/9Dm8ek
- Zhang S, Zhang Z, Shi W, Eremin SA, Shen J. Development of a chemiluminescent ELISA for determining chloramphenicol in chicken muscle. J. Agric. Food Chem. 2006; 54: 5718-5722. https://goo.gl/yEE33Z
- Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction technologies for medicinal and aromatic plants. International Centre for Science and High Technology. 2008. https://goo.gl/PQs6Lg
- Kornbery A. Why purify enzyme? In: methods in enzymology. Academic press. New York. 1990; 182: 1-5.
- Kim YH, Young JY. Peroxidase production from carrot hairy root cell culture. Enzyme and Microbial Technology.1996; 18: 531-535.
- Niraj KK, Fisseha K, Shishay N, Hagos Y. Welfare assessment of working donkeys in Mekelle city, Ethiopia. Global Veterinaria. 2014; 12: 314-319. https://goo.gl/AYg8wa
- Ila B, Rita M. Evaluation of peroxidases from various plant sources. International Journal of Scientific and Research Publications.2012; 2: 2250-3153. https://goo.gl/7XL9ng
- Wang MH, Lee SY, Rhee HI. Properties of anionic peroxidases purified from Chinese cabbage roots. Plant Physiology and Biochemistry. 1999; 37: 459-466. https://goo.gl/1hL8Ki
- Evans JJ. Peroxidases from the extreme dwarf tomato plant; Identification, isolation, and partial purification. Plant Physiol.1968; 43: 1037-1041. https://goo.gl/vy6u2s
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. The J Biol Chem. 1949; 177: 751-766. https://goo.gl/YicFaM
- Agostini E, Medina MI, Silvia R, Forchetti M, Tigier H. Properties of two anionic Peroxidase isoenzymes from Turnip (Brassica napus) roots. Journal of Agricultural and Food Chemistry.1997; 45: 596-598. https://goo.gl/Uz6jQi
- Kokkinakis DM, Brooks JL. Tomato peroxidase: purification, characterization, and catalytic properties. Plant Physiol.1979; 63: 93-99. https://goo.gl/qajBep
- Elisete S, Euclides J, Lourenco L, Valdir AN. Soluble and bound peroxidases from papaya fruit. Phytochemistry. 1990; 29: 1051-1056. https://goo.gl/z2eBji
- Voet D, Voet J. Biochemistry. John Wiley and Sons. New York. 1990; 1223. https://goo.gl/vn96tV
- Evans JJ, Alldridge NA. The distribution of peroxidases in extreme dwarf and normal tomato (Lycopersicon esculentum mill). Phytochemistry. 1965; 499: 499-503. https://goo.gl/54oiuK
- Ghaemmaghami F, Alemzadeh I, Motamed S. Seed coat soybean Peroxidase: Extraction and biocatalytic properties determination. Iranian Journal of Chemical Engineering. 2010; 7: 29. https://goo.gl/MzvAFh
- Ogana J, Eze S OO. Partial purification and characterization of peroxidase extracted from gongronema latifolium. American-Eurasian Journal of Scientific Research.2015; 10: 221-227. https://goo.gl/3VwR3N
- Rehman K, Yaqub M, Sheikh MA, Arshad M. Extraction and evaluation of peroxidase from vegetable sources. International Journal of Agriculture and Biology.1999; 3: 170-173.
- Tabatabaie M, Azdi Y, Khaleghparast S, Nayebpour SM. Purification and partial characterization of peroxidases from cultivated raphanussativus l. var. cicil. Med J Islam Repub Iran. 1998; 12: 1377. https://goo.gl/ANkJ5o
- Sariri R, Aghamaali M. Orange wastes as a natural source of peroxidase: extraction and purification. Pharmacologyonline. 2009; 3: 932-937. https://goo.gl/bgA8i8
- Mamounata D, Brice N, Ayekoue C, Dibala I, Soumaila D. Purification and characterization of sweet potato (Ipomoea batatas) peroxidase. Journal of Animal and Plant Sciences. 2014; 22: 3419-3432. https://goo.gl/CRhM36

Sign up for Article Alerts