An Evolving Companion Animal Health Sector in the United States
Rajashree Patil*
Domain Consultant, Veterinary Writing, Tata Consultancy Services, Pune, India
*Address for Correspondence: Rajashree Patil, Tata Consultancy Services, Sahyadri Park, Rajiv Gandhi Infotech Park, Hinjewadi, Pune, Maharashtra 411057, India, Tel: +919-011-010-341; E-mail: [email protected]
Submitted: 01 August 2018; Approved: 21 August 2018; Published: 22 August 2018
Citation this article: Rajashree P. An Evolving Companion Animal Health Sector in the United States. Int J Vet Sci Technol. 2018;2(1): 020-025.
Copyright: © 2018 Rajashree P. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Companion animals; FDA; CVM; Crossover drugs; Veterinary; Green book; Federal register notice
Download Fulltext PDF
In the United States (US), companion animals are categorized as major species and include dogs, cats, and horses. The US companion animal health sector is on a growth trajectory, driven by the rising popularity of pet ownership and the introduction of healthcare innovations converging with diverse therapeutic indications. Over the last decade, the introduction of drug crossovers from the human to animal healthcare sector has been increasing, especially for dogs and cats. Despite relatively lower population of companion animals compared to production animals, the trend has been to focus on this sector for the top pharma players, leading to an increase in product approvals. A trend of emergence of veterinary specific drugs has also been noticed in past few years. This article reviews various aspects of the companion health sector, including: key growth drivers, introduction of niche therapeutic targets, increasing drug crossovers, veterinary specific drugs, the companion animal drug development process, and high cost of animal drugs.
Introduction
The global animal health market supports a rapidly flourishing industry catering to the needs of several companion (e.g. dogs, cats, and horses) and production (e.g. cattle, pigs, sheep, poultry etc.) animal species. In recent years, leading animal pharma players are increasingly emphasizing development of healthcare products for companion animals, especially for dogs and cats. The companion animal healthcare sector has witnessed scientific innovations and technological advances such as, monoclonal antibody therapies, therapeutic vaccines, anti-neoplastic drugs, genomic tests, digital technologies, etc. over the past few years.
The US companion animal healthcare market valued at $ 14.36 billion in 2018 is expected to reach $ 19.40 billion by the end of 2023, exhibiting growing potential, at a compound annual growth rate (CAGR) of 6.20% [1].
Over past three years, 39 healthcare products targeted for the production animal sector were approved in the US wherein 34 drugs were approved for the companion animals (Table 1) [2]. Moreover, during the past two decades, out of 7 animal healthcare blockbuster drugs (annual sales greater than $100 million), 5 were targeted for companion animals [3].
An accelerated growth trend observed in the companion animal health sector is explained by a rising number of pets in the US, increased willingness of owners to pay for quality treatment, increased life span of pets introducing age related disease targets such as osteoarthritis, cancers, obesity etc. Moreover, the relatively lower cost and time required to develop drugs for the companion animal segment, and the greater market potential compared with the production animal segment are further stimulating this change. A trend of emergence of veterinary specific drugs has also been noticed in past few years.
Global animal healthcare sector
The global animal healthcare market is a highly consolidated market where the top ten players represent 86% of the market share [4]. Over the time the global animal healthcare sector has witnessed a lot of transformation. The top players in human pharmaceutical sector viz., Pfizer, Novartis, Elli Lilly, Sanofi, and Johnson & Johnson were involved in the merger and acquisition activities during past decade [5].
The major spin-offs or acquisitions were Elanco acquired Johnson & Johnson’s Janssen AH and Novartis AH in 2011 and 2015, respectively. Pfizer spun off its animal health business Zoetis in 2013 whereas Boehringer Ingelheim traded its consumer health business for Sanofi’s Merial AH, in 2016, thus positioning second largest company in global animal healthcare industry [6]. And most recently i.e. in July 2018, Eli Lilly has announced to spin-off its Elanco Animal Health business in order to focus on human health.
These consolidations and segregations of animal healthcare from parent human enterprises are powering both human and animal sectors in getting more clear focus on their investment landscapes, helping in minimizing the internal competition for resources, and are promoting faster decision making. History has revealed that post spun-off from Pfizer, market value of Zoetis has nearly tripled [7].
Facts and trends in the US companion animal healthcare sector
As the global economy is booming and population wealth is growing, there has been an increasing interest in pet ownership. Moreover, factors like changes in population and lifestyles, increased urbanization, nuclear families, single status, few or no children, and employment stresses are creating a greater need for mental, physical, and social support. These demands are strengthening the human-animal bond as a way to boost the health and emotional welfare of individuals and provide a safety support [8]. According to Dr. Alan Beck ‘The companionship of animals decreases loneliness, stimulates conversation, and improves feeling of wellbeing [9].
The American Pet Production Association’s (APPA) National pet owners survey, 2017-2018 reports that the US is home to 89.7 million dogs; 94.2 million cats; and 7.6 million horses. Approximately, 68% of US households own pets, which is equal to 84.6 million homes, out of which 60.2 million households own dogs, 47.1 million houses own cats, and 2.6 million houses own horses [10].
With, rising companion animal ownership, owners are increasingly aware of pet health and nutrition and owners are routinely seeking veterinary services. This in turn is creating a growing demand for diversified companion healthcare products e.g. drugs with varied therapeutic indications, feed additives, vaccines, and nutritional supplements. During 2017, out of the total $ 69.51 billion US pet industry expenditures, $ 32.18 billion were spent on veterinary care and medicines [10].
Introduction of niche markets to the companion animal healthcare industry
Techno-scientific advances, including improvements in nutrition and disease treatments, have resulted in an increase in the average life span of companion animals [11]. The companion animal ageing has led to an increased risk for age related disorders including: tumors, obesity, osteoarthritis, cardiovascular diseases, diabetes mellitus, hepatic, and renal diseases [12]. Pet demographic data from American Humane Association (AHA) published in the year 2011 depicts obesity in 56% of dogs and 54% of cats. The incidence of diabetes is increasing in both dogs (up 32%) and cats (up 16%) since the year 2006 and cancer is the most frequent cause of death in dogs over the age of two years [13].
Age-related disorders are providing niche markets for the animal health industry. Additionally, a condition like obesity serves as a parallel between companion animals and their owners, and opening an opportunity for crossover of the health products from human medicine to companion animal medicine [14]. These crossover indications have become a focus area for many top players in the animal health sector, including: Zoetis, Elanco, VetDC, and Merck.
According to data published in the FDA’s Green Book on approved animal drug products, out of the 34 new drug approvals (NADA or ANADA) in the companion animal segment between January 2016 and July 2018, 9 were targeted for niche indications i.e. osteoarthritis, hypertension, weight management, lymphoma, thyroid dysfunction, and Addison’s disease (Table 1) [2].
Crossover of human drugs to the companion animal healthcare sector
Out of the top five global animal healthcare players viz., Zoetis, Boehringer Ingelheim Animal Health, Merck Animal Health, Elanco, and Bayer Animal Health, four are divisions or subsidiaries of the big human pharma companies [15]. The strategy of sharing drug pipelines, resources, and intellectual properties, within the human and animal health divisions within the company, leveraging “drug crossovers”. Drug crossover has provided an opportunity to extend the opportunities of human drugs to the companion animal health segment [16].
Human drugs used in animals are commonly referred to as ‘crossover drugs’. A few smaller market players, like Kindred, are ‘petifying’ human drugs i.e. working with off patent human drugs to develop animal drugs [17]. Another player in the sector, VetDC is working to transform experimental human cancer drugs as options for pet cancer treatments [18]. Many ailments, including hormonal imbalances, cancers, diabetes, and obesity, can afflict both companion animals and human beings. Clinical diagnostic tools, including hematology, clinical chemistry, X-rays, ultrasonography, and Magnetic Resonance Imaging (MRI), are also applicable to both human and companion animal disease diagnosis and management, and frequently can be performed using the same instruments. Whilst studies performed during the pre-clinical development of human drugs, including research in laboratory animals on drug efficacy, safety, and pharmacokinetics; can be of direct value in assessing use of these drugs in dogs and cats. Therefore, pharma companies with both animal and human health divisions may gain some advantage from availability of primary animal safety data required for veterinary submission. This enables the sponsor in getting access of human drugs to veterinary market, by putting some extra money to generate additional prerequisite data [19].
Animal specific drugs
Animal specific drugs were uncommon in veterinary industry, however a recent trend of emergence of animal specific treatments have been noticed especially for the treatment of companion animals. To list a few, the drugs like Maropitant citrate (acute vomition), Sarolaner (oral anti-ecto-parasitic), Fluralaner and Lotilaner (topical anti-ecto-parasitic), Alfaxalone (Anesthetic), Eprinomectin (oral anti-endo-parasitic) have been approved specifically to treat dogs and cats, during past 3 years (Table 1) [2].
Drug development process for companion animal drugs
All drugs targeted for companion animal use, except for topical anti-parasitic drugs (regulated by the Environmental Protection Agency or EPA) are regulated by the US Food and Drug Administration (FDA) under the Federal Food, Drug and Cosmetic Act (FFDCA) [20].
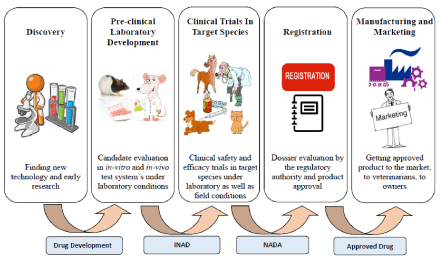
Upon getting a promising test drug post completion of early drug discovery and development, and converging on factors like market demands, patentability, availability of alternatives in the market, manufacturing feasibility etc., the sponsor takes a business decision to advance the lead molecule to more expensive registration phase by filing for Investigational New Drug Application (INAD) and further conducts studies to generate data on safety, efficacy and quality of test drug. These data form the basis for New Animal Drug Application (NADA) which is reviewed by the FDA’s Center for Veterinary Medicine (CVM).
The drug dossier for animal drug is composed of seven technical sections, out of which five are major viz., target animal safety, effectiveness, human food safety (not as important for companion animal drugs), chemistry, manufacturing, controls, and environmental assessment, whereas two are minor technical sections viz., product labelling and all other information. CVM thoroughly reviews each technical section data and if found suitable, grants marketing approval within the US territory (Figure 1) [21].
To generate safety data, the sponsor has to perform Target Animal Safety (TAS) studies in the target species. Additionally, the sponsor may need to conduct special studies e.g. GI endoscopy, specific breed safety (e.g. Collie safety) study, handler safety study, reproductive toxicity, ocular toxicity, genotoxicity etc. in the laboratory animals or in cell based test systems in a controlled environment. Field safety studies under the natural conditions also need to be conducted.
For assessment of effectiveness of the test drug, the sponsor needs to conduct studies using cell and laboratory animal based disease models; pharmacokinetic studies; dose determination and confirmation studies in target species under controlled conditions; and finally field efficacy trials under the natural conditions.
Under drug quality section, the sponsor needs to generate data pertaining to the ingredients and their sources, manufacturing, packaging, storage, expiry date, and stability etc.
Along with safety, efficacy, and quality data, the sponsor has to compile the product labelling data along with all other information such as literature support for the test drug, foreign market experience, drug class information, and human approval data, if any.
Human food safety studies are not required for companion animal drugs as food products are not derived from them. In addition, the sponsor can apply for a waiver-off for environmental safety assessment studies by applying for ‘Categorical Exclusion’ as the drugs indicated for companion animals, unlike production purpose animals are primarily intended for individual animal treatment and not for herd use, restricting drug entry into the environment. Conducting human food safety studies and environmental assessment tests consumes lots of money and time and as the companion animal drug development does not mandates to perform these tests; it reduces the financial as well as temporal burden.
Upon generation of all the pre-requisite data on each of the section, the sponsor has to submit it to CVM in a phased manner for approval and after completion of all technical sections the sponsor can apply for administrative NADA [22]. Post approval of administrative NADA, FDA announces the approval status through Federal Register Notice and the information gets added to the Code of Federal Regulations and the Green Book [2].
High cost of animal drug approval and drugs
Animal drug development is a complex, time consuming process which can take up to 10 years for approval and demands an investment approximately up to $ 100 million [23]. Animal drug developer has to bear not only the cost of research and development, manufacturing, and marketing but also the post marketing safety evaluation expenses for the drug candidates which continues throughout entire lifetime of the drug candidate. In return the animal drug developer gets only a limited period of patent protection to recoup their investment. In contrast, the cost of human drug development is greater (around $ 500 million), at the same time they have advantage of huge market and more returns.
Considering these facts, although the worldwide sales of animal healthcare products for companion animals have exceeded about $ 8 billion annually, for getting state-of-the-art remedies to treat their pets, owner has to pay a hefty sum.
More recently third party payers like insurance companies have penetrated the US pet industry providing further support for treatment costs leading to a potential boosts the prices of companion animal drugs.
Conclusion
The US companion animal health sector is evolving to develop and register more intricate and diversified therapeutic indications. Newly emerging diseases in companion animals are creating a demand from veterinarians and pet owners for faster access to medicines. This sector is mirroring many aspects of the human health industry, and there is an emphasis on novel animal specific remedies and innovative drug delivery solutions. Additionally as US has captured major market share of the global animal health market (47%), followed by Europe (32%), and the rest of the world (21%), the dynamics (innovations, trends, growth drivers, challenges etc.) of the US animal healthcare sector are getting percolated among rest of the world geographies.
To tackle all the demands of the booming industry and to facilitate the journey of innovations from lab to veterinarian’s clinic, drug developers need to have a focused planning, thorough understanding of the companion animal disease targets, and a precise regulatory strategy to manage the drug development process. Cultivating strategic partnerships with regulatory consultancies that have a sound knowledge of the companion animal health sector, a thorough understanding of the regulatory requirements, a wide therapeutic area experience, and a rich network of resources within the sector can facilitate the early penetration of new drugs into this growing market.
Acknowledgments
I am thankful to Dr. Rajesh Pandey, Offering Lead and Domain Consultant, Medical Writing, Tata Consultancy Services, Mumbai for critically reviewing this manuscript and providing valuable inputs.
Author Information
Rajashree Patil is a veterinarian and holds a Master’s Degree in Veterinary Pharmacology and Toxicology. Presently she is associated with Tata Consultancy Services, India as Domain Consultant for Veterinary Writing wherein she is responsible for the regulatory and publication writing services for the veterinary medicinal products.
- Companion animal health care market by market data forecast. Global. 2018; 182. https://goo.gl/6gQMxZ
- Federal register. New animal drugs; approval of new animal drug applications. Food and Drug Administration. 2018. https://goo.gl/7PR56g
- Evans T, Chapple N. The animal health market. Nature Reviews Drug Discovery. 2002; 1: 937-938. https://goo.gl/zT6qhs
- Dimitri D, Williams B, Div K. Animal health strategy playbook for an evolving industry. PwC Analysis. 2015.
- Sam Al-Murrani. Consolidation in animal health: A Much-Needed Growing Pain. International Animal Health Journal 20. 2018.
- Cathy Yarbrough. Why animal health is the next big growth area. Life Science Leader. 2016. https://goo.gl/y7PvJi
- Lilly plans Elanco IPO, posts quarterly profit above estimates. Business news. 2018. https://goo.gl/P3B61g
- Marguerite O'Haire. Companion animals and human health: Benefits, challenges, and the road ahead. Journal of Veterinary Behavior. 2010; 5: 226-234. https://goo.gl/KbY2ej
- A scientific look at the human-animal bond, at inaugural educational summit-think paw sitive. 2002. https://goo.gl/EPVH28
- APPA - Pet industry market size & ownership statistics. American Pet Products Association. 2018. https://goo.gl/dF15zN
- Rick D. Extending pet longevity: Our companions in sickness and in health. The Futurist. 2014; 47-51. https://goo.gl/PEbRN8
- Emi K and Catherine R. Age at diagnosis of select chronic diseases. Veterinary Focus. 2015; 1: 28-30.
- U.S. Pet (dog and cat) population fact sheet. American Humane Association. 2018. https://goo.gl/jp8kLp
- Heuberger R, Wakshlag J. Characteristics of ageing pets and their owners: dogs v. cats. British Journal of Nutrition. 2011; 106: 150-153. https://goo.gl/SBNLxr
- Animal Health in 2017. A year when antibiotic alternatives were pushed into the spotlight. Animal Pharm. 2018. https://goo.gl/1a1DNV
- Human drugs for veterinary use - Current trends and future commercial prospects for crossover drugs. Research and Markets. 2018. https://goo.gl/FyS2dn
- Victoria C. 'Petifying' human drugs a growing market. San Francisco Chronicle. 2014. https://goo.gl/7YtuUs
- About VetDC. 2018. https://goo.gl/w6zbxs
- Claire ML. Challenges and opportunities in animal drug development: A regulatory perspective. Nature Reviews Drug Discovery. 2003; 2: 915-919. https://goo.gl/sesByw
- Ashley S. FDA’s role in animal health Yes! No! Maybe So! - What FDA does and does not regulate. FDA Veterinarian Newsletter. 2010; 3.
- Rajashree P, Chandrakant G. Regulatory framework for approval of new animal drugs in United States. International Journal of Advanced Veterinary Science and Technology. 2017; 1: 316-322. https://goo.gl/QzhLpz
- From an idea to the marketplace: The journey of an animal drug through the approval process. U.S. Food & Drug Administration - Animal and Veterinary. 2018. https://goo.gl/sjRnNk
- The balance, Biotech industry - Sales and marketing. Animal Pharmaceutical Companies. 2018. https://goo.gl/SDa1v7

Sign up for Article Alerts