A Review on Avian Influenza and its Economic and Public Health Impact
Mullusew Gashaw*
Livestock Resource Development and Promotion, North Wollo Zone Dawunt District, Addis Ababa Unversity, Ethiopia
*Address for Correspondence: Mullusew Gashaw, Livestock Resource Development and Promotion, North Wollo Zone Dawunt District, Addis Ababa Unversity, Ethiopia, Tel: + 0941-626-272; E-mail: gashawmuller@gmail.com
Submitted: 19 March 2020; Approved: 29 July 2020; Published: 31 July 2020
Citation this article: Gashaw M. A Review on Avian Influenza and its Economic and Public Health Impact. Int J Vet Sci Technol. 2020;4(1): 015-027.
Copyright: © 2020 Gashaw M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Avian influenza; Economic risk; HPAI; Public health risk
Download Fulltext PDF
Avian influenza, also known as avian flu or bird flu, is an infectious disease of birds caused by type a strains of the Influenza virus with a world wide distribution. Avian influenza A virus is a zoonotic pathogen with a natural reservoir entirely in birds. In poultry, it is unusual in that it can cause a range of disease symptoms from a subclinical infection to being highly virulent with 100% mortality. In the natural environment, it is generally spread by ingestion or inhalation. Virus-laden feces and respiratory secretions present on fomites are effective means of transmitting the virus. Airborne dissemination is also an important means of transmission. Strains are classified into low and high pathogenic types depending on the severity of diseases which they cause. The viruses are now widely recognized as important threats to agricultural biosecurity and public health, and as the potential source for pandemic human Influenza viruses. HPAIV is one of the most significant pathogens of agricultural concern worldwide, mainly because of the potential economic impact of HPAI. The economic consequence of HPAI outbreaks is severe due to the cost of culling and bird replacement, loss of customer confidence, local and international trade losses, the cost of biosecurity, and the cost for veterinary and infrastructure improvement. The magnitude and duration of these events will ultimately determine the overall impact to the economy. In Ethiopia, there is a pandemic threat because of its wetland areas that are visited by migratory birds and illegal trade. A definitive diagnosis of AI is established by direct detection of AI viral proteins or genes in specimens such as tissues, swabs, cell cultures, or embryonated eggs and isolation and identification of AI virus or by a molecular detection. There is no effective treatment for avian influenza. However, good husbandry, proper nutrition, and broad spectrum antibiotics may reduce losses from secondary infections. Therefore, timely development of an effective influenza vaccine must and should be made a public health priority, and biosecurity measures can be established to prevent interaction of wild birds and domestic poultry, thereby reducing the risk of AI virus introduction into domestic poultry.
Introduction
The rapid spread of infectious disease greatly influences poultry production. Fast and easily disseminating diseases threatening poultry raised under production systems includes marek’s disease, infectious bursal disease, avian influenza and other respiratory disease. Therefore, the presence of highly virulent strains of infectious agents make poultry production a risky business and further limit the development and the contribution of the sector to the country economy [1].
Avian influenza is one of the most devastating disease or asymptomatic infection caused by viruses in the family Orthomyxoviridae, genus Influenza virus A, which contains a genome composed of eight segments of single-stranded negative-sense Ribonucleic Acid (RNA). AI viruses are sub typed by their surface Hemagglutinin (HA)and Neuraminidase (NA) glycoproteins, which are major determinants of the pathogenicity, transmission, and adaptation of the AI virus to other species, but these three traits plus infectivity, are multigenic [2].
Wild water birds are the natural reservoir hosts of avian Influenza viruses, and in these species infection typically causes little or no disease because influenza A viruses co-exist in almost perfect balance with the hosts [3]. The viruses are important pathogens for humans and animals alike. In humans, influenza is a highly contagious respiratory disease which, in most cases, is self-limiting but nevertheless causes substantial morbidity and mortality worldwide [3,4].
When an avian Influenza virus (usually of subtype H5 or H7) is transmitted from reservoir hosts to highly susceptible poultry species such as chickens and turkeys, generally it initially induces only mild disease, termed Low Pathogenic Avian Influenza (LPAI). However, in cases where the particular poultry species supports several sequential cycles of infection, these strains may undergo a series of mutation events resulting in adaptation to their new hosts, and the virus may switch into a highly pathogenic form (highly pathogenic avian influenza, HPAI) [5]. HPAI in poultry is characterized by sudden onset, severe illness of short duration, and mortality approaching 100 % in susceptible species. Due to heavy commercial losses to the poultry industry, HPAI attracts considerable attention. Because of their potential to give rise to HPAI viruses, LPAI viruses of subtypes H5 and H7 are also notifiable disease agents at international level [5].
The global publicity surrounding the impacts of the H5N1 highly pathogenic avian Influenza virus has fostered wide public recognition of the potentially serious economic and public health impacts of avian influenza outbreaks [6] and it is identified as a source for pandemic human Influenza viruses that could have severe economic and public health impacts on countries worldwide [7].
A definitive diagnosis of AI is established by direct detection of AI viral proteins or genes in specimens such as tissues, swabs, cell cultures, or embryonating eggs or by isolation and identification of AI virus [8] or by a molecular detection. Presently, no practical, specific treatment exists for AI virus infections in commercial poultry [9] and antiviral resistance is an increasingly important issue because human avian influenza vaccines are not yet widely available, and treatment of human infections is currently limited to supportive therapy and treatment with antivirals [10,11].
The avian Influenza virus is hard to control because of the frequent contact with chickens, ducks in the live poultry markets and the birds in our daily life. In addition, the migration of the wild birds from one area to another every year in the world made the viruses transmit more in the world [12]. Important factors often overlooked in avian influenza risk analyses are that vaccination, and concurrent infection by low-pathogenic avian Influenza viruses, do not prevent poultry from becoming infected with the H5N1 highly pathogenic Influenza virus but can prevent poultry infected from exhibiting disease symptoms or mortality [13].
Therefore, the objectives of this seminar paper are:
➣ To make an overview on the general accounts (mainly occurrence, mode of transmission, control and prevention) of avian influenza and
➣ To briefly point out the public health (zoonotic) and economic importance of avian flu.
Material and Metho
History
The most widely quoted date for the beginning of recorded history of avian influenza (initially known as fowl plague) was in 1878 when it was differentiated from other diseases that caused high mortality rates in birds. Initially, the disease was confused with the acute septicemic form of fowl cholera until 1880. In 1901, the cause was a filterable agent, but the virus was not identified nor classified as an Influenza virus until 1955 [14]. Until 1950s fowl plague, however, also included Newcastle disease. Between 1959 and 1995, there were 15 recorded occasions of the emergence of HPAI viruses in poultry, but losses were minimal. Between 1996 and 2008, however, HPAI outbreaks in poultry have occurred at least 11 times and 4 of these outbreaks have involved millions of birds [15].
Before the 1990s, HPAI caused high mortality in poultry, but infections were sporadic and contained. Human infections were first reported in 1997 in Hong Kong [16]. Since 2003, more than 700 human cases of Asian HPAI H5N1 have been reported to the WHO, primarily from 15 countries in Asia, Africa, the Pacific, Europe, and the Middle East, though over 60 countries have been affected [15,16].
Etiology
Avian influenza results from infection by viruses belonging to the species influenza A virus, genus Influenza virus A and family Orthomyxoviridae. The viruses are also called type A Influenza viruses. influenza A viruses are further classified into subtypes based on two surface proteins, the Hemagglutinin (HA) and Neuraminidase (NA) [17]. At least 16 hemagglutinins (H1 to H16), and 9 neuraminidases (N1 to N9) have been found in viruses from birds, while two additional HA and NA types have been identified, to date, only in bats [17,18]. The viruses are roughly spherical (120 n M) with glycoprotein spikes on the surface and genome consisting of also eight RNA fragments that encode 10 proteins. The Haemagglutinin (HA), Neuraminidase (NA) and Matrix (M2) proteins are embedded in the envelope lipid bilayer derived from the host cell [19] (Figure 1). The M1 protein underlying the envelope is the major determinant of virion morphology [20].
The Nucleoprotein (NP) associates with each RNA segment to form the Ribonucleoprotein (RNP) complex, which also contains small amounts of the three polymerase subunits. The nonstructural proteins NS1 and NS2 are found only in infected cells [19]. The surface glycoproteins HA and NA are critical for the biology of Influenza virus. HA is responsible for the virus attachment to the cell surface, binding to sialic acid residues in cell membrane glycoproteins, thus triggering viral fusion and entry [21] whereas the function of neuraminidase is to release newly formed viruses [8]. Therefore, they constitute an important molecular determinant of host range and tissue pathology [22].
The RNA-dependent RNA polymerase and the NS1 proteins of Influenza virus also are determinants of viral pathogenicity and host range [23]. The standard nomenclature for Influenza viruses includes the influenza type, the host of origin (excluding humans), the place of isolation, the strain number, the year of isolation, and finally the influenza A subtype based on H and N antigens in parentheses (e.g. A /Duck/ Vietnam/11/04 (H5N1)) [24].
antigenic drift and shift: influenza A viruses are very diverse, and two viruses that share a subtype may be only distantly related. The high variability is the result of two processes, mutation and genetic reassortment [25]. These viruses lack a proofreading and error correction mechanism during replication, therefore small, constant genetic changes occur due to point mutation, deletion, or substitution, which can result in new mutants [26]. In case selective pressures (such as neutralizing antibodies, suboptimal receptor binding or chemical antivirals) are acting during viral replication on a host or population scale, mutants with corresponding selective advantages (e.g. escape from neutralisation, reshaped receptor-binding units) may be singled out and become the dominant variant within the viral quasispecies in that host or population. If antigenic determinants of the membrane glycoproteins HA and NA are affected by mechanisms driven by immunity, such a (gradual) process is referred to as antigenic drift [27].
antigenic drift, in contrast, denotes a sudden and profound change in antigenic determinants, i.e. a switch of H and/or N subtypes, within a single replication cycle. This occurs in a cell which is simultaneously infected by two or more influenza A viruses of different subtypes [7,28].
Host range: Avian Influenza viruses can infect commercial and non-commercial poultry, indoor and outdoor reared poultry, pet birds, wild birds, and a variety of other avian and non avian species [8]. Wild aquatic birds, notably members of the orders Anseriformes (ducks and geese) and Charadriiformes (gulls and shorebirds), are carriers of the full variety of Influenza virus A subtypes, and thus, most probably constitute the natural reservoir of all influenza A viruses [29]. Although aquatic birds are the reservoir for all recognized influenza A subtypes, some viruses have also been detected in other species. However, there appears to be a substantial degree of species specificity. For example, H1N1 and H3N2 have been detected in swine, whereas H3N8 and H7N7 are found in horses [30]. Recently, the horse H3N8 virus jumped the species barrier to cause infections and initiate an evolving outbreak in canines [31].
In addition, while all bird species are thought to be susceptible, some domestic poultry species chickens, turkey, guinea fowl, quail and pheasants are known to be especially vulnerable to the sequelae of infection [32]. For instance, the vast majority of LPAI viruses are maintained in asymptomatic wild birds, particularly birds in wetlands and other aquatic habitats [33]. When these types of viruses from wild birds are transferred to poultry, the viruses may circulate inefficiently and die out; become adapted to the new host and continue to circulate as LPAI viruses; or if they contain H5 or H7, they may evolve into HPAI viruses. In contrast, HPAI viruses are not usually found in wild birds, although they may be isolated transiently near outbreaks in poultry [34].
Geographic distribution: Avian Influenza (AI) virus is a global virus that knows no geographic boundaries, has no political agenda and has been isolated from poultry, captive birds, and wild birds in Africa, Asia, Australia, Europe, and North and South America, and anti-AI antibodies have even been identified in Antarctic penguins [8]. For instance, some noteworthy features of the ongoing outbreaks of infection with highly pathogenic H5N1 in Asia, Europe, Africa, and the Middle East are the number of affected animals and the widening geographic spread [35] (Figure 2). Although human activities may contribute to the geographic spread of H5N1, migratory birds may also play an important role, as is suggested by outbreaks in migratory birds in remote areas such as Mongolia [36]. LPAI viruses are cosmopolitan in wild birds, although the specific viruses differ between regions [37]. Annual outbreaks of influenza occur regularly in temperate regions of the world with remarkable seasonality, defined by peak incidence in the colder months of the year. Such annual outbreaks vary in severity [38].
The occurrence of AI epidemic in Africa is due to the flyways of migratory birds, which link the endemic and newly infected countries with free areas on this continent, and because of the risk of introduction through legal and illegal trade [39]. From the sub-Saharan countries, Ethiopia and Kenya have not yet experienced any outbreaks, but the virus has been circulating in neighboring countries, e.g. Sudan, and it could enter these countries through various pathways, including illegal bird trade. However, Ghana and Nigeria have both experienced several outbreaks and are on the same bird flyways [40].
Source of infection: Aquatic wild birds are the natural reservoir of influenza A viruses and are thought to be the principal source of viral spread to other species [30]. The virus replication is primarily limited to the epithelial cells of the intestinal tract [41,42], and infected birds remain asymptomatic but shed the virus into the environment via feces, and less frequently saliva and nasal secretions [43] (Figure 3). Sources of infection may also include other animals like swine [44].
Mode of transmission between birds: As the natural reservoir for AI, infected waterfowl are believed to pass the AI virus to domestic poultry by the fecal-oral route through contaminated water sources, feed, and housing facilities/ shared environment, where infectious virus can be excreted by birds for up to 10 days. The virus can survive at 4oC in feces for at least 35 days [45,46]. Transmission of influenza A viruses from aquatic birds likely occurs through shared water source and infected migratory birds may result in wide geographic spread of viruses. Although flyways of bird populations are partially separated, allowing distinct gene pools of viruses to develop [30], evidence suggests that there is also interregional transmission by infected migratory birds. One study has demonstrated that H2 influenza A viruses isolated from shorebirds in North America between 1985 and 1998 contain genes belonging to a Eurasian lineage of H2 viruses [47].
Transmission is also possible via aerosols or airborne droplets, as well as by wind dispersion of fomites including contaminated dust. Indirect exposures occur through surface contamination of equipment, clothing, and shoes from dust, feces, secretions, and contaminated feathers [48]. Interspecies transmissions of AI viruses have also occurred in other species as well. [49].
Pathogenicity and pathogenesis
Avian Influenza viruses are classified as either low pathogenic (also called low pathogenicity) avian Influenza viruses or highly pathogenic (high pathogenicity) avian Influenza viruses and defined as HPAI or LPAI by its ability to cause severe disease in intravenously inoculated young chickens in the laboratory, or by its possession of certain genetic features that have been associated with high virulence in HPAI viruses (i.e., the sequence at the HA cleavage site) [50,51]. HPAI viruses usually cause severe disease in chicken and turkey flocks, while LPAI infections are generally much milder in all avian species [52].
Pathogenicity as a general viral property in influenza A viruses is a polygenic trait and depends largely on an optimal gene constellation affecting host and tissue tropism, replication efficacy and immune evasion mechanisms amongst others. In addition, host and species-specific factors contribute to the outcome of infection, which, after interspecies transmission, is therefore unpredictable [53]. LPAI viruses can be introduced by various pathways into poultry flocks. Following a variable and indecisive period of circulation (and, presumably, adaptation) in susceptible poultry populations, these viruses can saltatorily mutate into the highly pathogenic form [54].
First, in poultry, the process of pathogenesis begins by inhalation or ingestion of infectious LP or HPAI virions. Because trypsin-like enzymes in respiratory and intestinal epithelial cells allow cleavage of the surface hemagglutinin, multiple replication cycles occur in respiratory and/or intestinal tracts with release of infectious virions. Second, with HPAI viruses, after initial replication in respiratory epithelium, the virions invade the submucosa, entering capillaries. The virus replicates within endothelial cells and spreads via the vascular or lymphatic systems to infect and replicate in a variety of cell types in visceral organs, brain, and skin. Alternatively, the virus may become systemic before having extensive replication in vascular endothelial cells. The virus is present in the plasma, red and white blood cell fractions. Macrophages appear to play a role in systemic virus spread. The presence of a hemagglutinin proteolytic cleavage site that can be cut by ubiquitous furin like cellular enzymes is responsible for this pantropic replication. Clinical signs and death are due to multiple organ failure [8].
Third, for the LPAI viruses, replication usually is limited to the respiratory or intestinal tracts. Illness or death is most often from respiratory damage, especially if accompanied by secondary bacterial infections. Sporadically in some species, the LPAI viruses spread systemically, replicating and causing damage in kidney tubules, pancreatic acinar epithelium, oviduct and other organs with epithelial cells having trypsin-like enzymes. Pathogenesis of the infection process is less well understood in non-gallinaceous birds [8].
Clinical manifestation and pathological lesions in poultry
The incubation period of avian Influenza viruses in poultry can be a few hours to a few days in individual birds, and up to 2 weeks in the flock [33]. For mammals, the period is also thought to be short, and might be as little as 1-2 days in some cases [55]. Following an incubation period of usually a few days depending upon the characteristics of the isolate, the dose of inoculum, the species, and age of the bird, the clinical presentation of avian influenza in birds is variable and symptoms are fairly unspecific [56].
The symptoms following infection with low pathogenic AIV may be as discrete as ruffled feathers, transient reductions in egg production or weight loss combined with a slight respiratory disease [57]. In its highly pathogenic form, the illness in chickens and turkeys is characterised by a sudden onset of severe symptoms and a mortality that can approach 100% within 48 hours [53]. Often, only a section of a stable is affected. Many birds die without premonitory signs so that sometimes poisoning is suspected in the beginning [58].
Oedema, visible at feather-free parts of the head, cyanosis of comb, wattles and legs, greenish diarrhoea and laboured breathing may also be inconsistently present. In layers, soft-shelled eggs are seen initially, but any laying activities cease rapidly with progression of the disease [56]. Nervous symptoms including tremor, unusual postures (torticollis), and problems with co-ordination (ataxia) also dominate the picture in less vulnerable species such as ducks, geese, and ratites [59]. Microbiological features found in infected poultry are infarction of tissue and inflammation of inner organs which are generally found in brain, heart, lungs, pancreas, primary and secondary lymphoid organs [51].
Diagnosis
Clinical diagnosis: Clinically the disease is indistinguishable because lesions and symptoms are to variable and confuse with other diseases and AI virus cannot be diagnosed by clinical signs and symptoms alone. Therefore, confirmation should be undertaken by specialized laboratories, serology and virology are necessary [60].
Laboratory diagnosis: Avian Influenza viruses can be detected in oropharyngeal, tracheal and/or cloacal swabs from live birds, with differing recovery rates from each site depending on the virus, species of bird and other factors. Very small swabs can be valuable in small birds, but feces can be substituted if cloacal samples are not practical (e.g., can not be collected without harming the bird) [18]. Immature feathers may also be a useful sample (Nuradji et al., 2015). Samples from internal organs are also tested in dead birds suspected of having HPAI [50].
Virus isolation: virus isolation by inoculating the sample into hatching chicken eggs for detecting a property of red blood cells precipitation can be performed in all species, and can be useful for virus characterization [61] This technique is the “gold standard” but laborious and time insensitive, used primarily for diagnosis of first clinical case and to obtain virus isolated for further laboratory analysis [62]. laboratory diagnosis of AI viruses can also be performed by serological test such as hemagglutinin inhibition test, Agar Gel Immune Diffusion (AGID), antigen-detection ELISAs or other immunoassays, or by a molecular test such as RT-PCR [30]. The viruses can be identified as influenza A viruses with hemagglutination inhibition test, in which the Hemagglutinin (HA) protein of avian influenza has the property to agglutinate erythrocytes from a number of species including horses. A specific antibody to the antigenic sites on the avian influenza HA molecule prevents or inhibits the hemagglutination reaction. Therefore, hemagglutination inhibition test can be used to type the patient antibodies to avian Influenza virus when standard avian influenza antigen is available as reference material [61].
Use of the AGID test to demonstrate nucleocapsid or matrix antigens is also a satisfactory way to indicate the presence of influenza A virus in amnioallantoic and chorioallantoic fluid, but various experimental and commercial rapid, solid-phase antigen-capture ELISAs (AC-ELISAs) are an effective alternative [63]. They use a monoclonal antibody against the nucleoprotein; they should be able to detect any influenza A virus. The main advantage of these tests is that they can demonstrate the presence of influenza A within 15 minutes. The disadvantages are that they may lack sensitivity, they may not have been validated for different species of birds, subtype identification is not achieved and the kits are expensive [64].
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) is another powerful technique for the identification of Influenza virus genomes and allow for sensitive and specific detection of viral nucleic acid [24]. RT-PCR techniques on clinical specimens can, with the correctly defined primers, result in rapid detection and subtype identification (at least of H5 and H7), including a DNA product that can be used for nucleotide sequencing [65]. However, the preferred molecular detection tests for influenza A virus is the real-time RT-PCR, a modification to the RT-PCR that reduces the time for both identification of virus subtype and sequencing [66]. A disadvantage of RT PCR methods is its proneness for contamination and the consequent risk of false positive results [24].
Differential diagnosis: Diseases must be considered in the differential diagnosis of HPAI because of their ability to cause a sudden onset of disease accompanied by high mortality or haemostasis in wattles and combs are velogenic Newcastle disease, infectious laryngotracheitis (chickens), duck plague, acute poisonings, acute fowl cholera (pasteurellosis) and other septicaemic diseases, bacterial cellulitis of the comb and wattles [56]. For LPAI viruses, other causes of respiratory disease and drops in egg production must be investigated such as lentogenic Newcastle disease virus, avian pneumovirus and other paramyxoviruses, infectious laryngotracheitis, infectious bronchitis, chlamydia, mycoplasma, and various bacteria [9].
Impact 0f the Disease
Public health impact
Outbreaks of HPAI viruses in wild and domestic birds are rare, but once infection occurs it can be serious from veterinary, medical, and public health perspectives [67]. The two most commonly reported avian Influenza viruses from human clinical cases have been the Asian lineage H5N1 HPAI viruses, and recently, H7N9 LPAI viruses in China [68,69]. There are currently no reported human infections caused by Asian lineage H5N8 viruses, although four infections with H5N6 viruses have been detected in China since 2014 [70,71].
Illnesses caused by other subtypes have also been reported sporadically, with documented clinical cases caused by H9N2 (Eurasian lineage), H6N1 and multiple H7 and H10 avian Influenza viruses [72,73]. Whether these infections are truly less common than subtypes such as H5N1 is unclear: viruses that tend to cause milder illnesses (e.g., H9N2 viruses) are less likely to be identified than those causing severe disease. Serological surveys in some highly exposed populations suggest the possibility of low level exposure to HA types found in birds, including H4, H5, H6, H7, H9, H10, H11 and H12 [74]. Adaptation to humans is possible, though rare, and some previous human pandemics were caused by partially or wholly avian viruses [75,76].
In general, Influenza viruses exhibit host species adaptation with transmission occurring most frequently and with ease between individuals of the same species; occasionally interspecies transmission to closely related species occurs [77]. On rare occasions, AI viruses have exhibited interspecies transmissibility to humans [9]. Although rare, AI viruses or their genes have been transferred to humans: transfer of complete AI viruses with individual sporadic infections, and appearance of individual AI viral gene segments in pandemic human Influenza viruses (i.e., reassortment of gene segments) [8].
Human pandemic: Over the past 150 years at least four pandemics of avian influenza occurred at irregular intervals, including three in the 20th century. These have caused high attack rates in all susceptible age groups with high morbidity and mortality. Earliest pandemic occurred in 1918-19 and is widely known as "Spanish flu.” It was caused by (H1N1) and lead to the highest number of known flu deaths; more than 500,000 people died in the United States alone and 20 to 50 million may have died worldwide. Second was "Asian flu" which occurred in 1957-58. Its causative agent was (H2N2). It resulted in about 70,000 deaths in the United States. Third pandemic commonly called as "Hong Kong flu" was caused by (H3N2) in 1968-69. There were approximately 34,000 deaths in the United States. This virus was first detected in Hong Kong in early 1968 [78].
Since the outbreaks of H5N1 HPAI in poultry and humans in Hong Kong in 1997 and H7N7HPAI in the Netherlands in 2003, there have been concerns that AI viruses could persist in some poultry populations and emerge as a pandemic virus for humans through multiple mutations or reassortment [67]. The 2009 pandemic H1N1 was the result of a reintroduction of H1N1, which over time had mutated and reassorted influenza A genes from various avian, human, and swine Influenza viruses [79,80,81]. Human infection with avian influenza A (H7N9) was also first reported in China in March 2013, and since then hundreds of human cases have been confirmed. Today, AI viruses of concern as potential pandemic strains are some H5, H7, and H9 subtypes that have crossed the human species barrier multiple times to produce sporadic infections [82].
The current spread of avian influenza H5N1 in domestic poultry flocks and wild birds across the world, as well as the demonstrated ability of this virus to cross the species barrier and infect humans, has lead to a high level of concern that a pandemic may develop. For a pandemic to arise, three pre-requisites have been identified such as a new virus subtype to which the population has little or no immunity must emerge, the new virus must be able to replicate in humans and cause serious illness, and the new virus must be efficiently transmitted from one human to another [83].
Transmission to humans: The close proximity of birds to humans increases the risk of transmission to humans via aerosol or large airborne droplets, fecal contamination with dispersion via fomites, and direct contact with infected birds. Direct contact with infected poultry, or surfaces and objects contaminated by their faeces, is presently considered the main route of human infections. As infected birds shed large quantities of virus in their faces, opportunities for exposure to infected droppings or to environments contaminated by the virus are abundant under such conditions. Also, sick birds may be slaughtered for consumption in the developing world, leading to increased risk of exposure [84]. This is because of many households in developing countries depends on poultry for income and food, many families sell or slaughter them or even consume them when signs of illness appear in a flock [85].
In general, people at greatest risk for AI virus exposure and infection include farm workers, live bird market workers, butchers and home processors of poultry, hunters that slaughter, eviscerater, and defeather infected wild birds, and those preparing to cook contaminated meat getting viruses on their hands and transferring them by touching mucus membranes [86]. However, properly cooked and properly handled poultry and poultry products can safely be consumed [85].
H5N1 HPAI virus has expanded its host range, as it has infected dogs and other mammals through the consumption of uncooked infected poultry, wild birds, or their products [67,86]. This has raised concern that dogs and other pets also have the potential to be intermediate carriers that can transfer the H5N1 Influenza virus to humans. Although rare, evidence of direct human to human transmission of H5N1 associated with a poultry outbreak also occurred in Southeast Asia. Sustained human to human transmissibility of H5N1 HPAI would require genetic adaption of AI PB2 internal protein [87].
Clinical sign in humans: Most zoonotic infections caused by Asian lineage H5N1 HPAI viruses seem to become apparent within approximately 5 days, although the incubation period for some cases may be as long as 8 and possibly 17 days [88,89]. Estimates of the mean incubation period for the zoonotic H7N9 viruses have varied from 3 days (in two analyses, which considered large numbers of cases) to 5-6 days, with a range of 1-13 days [90,91].
Clinical symptoms of avian influenza infections in humans range from asymptomatic infection or mild conjunctivitis to fatal systemic disease and multiorgan failure including severe or fatal respiratory, gastrointestinal, or neurological syndromes [24,2]. Initial symptoms include headache, fever, fatigue, myalgia, odynophagia, cough and rhinorrhea, abdominal pain, vomiting, diarrhea, hepatic dysfunction, Reye's syndrome, pancytopenia, renal failure, pulmonary hemorrhage, acute respiratory distress syndrome and septic shock have been reported with varying frequency [92,93].
For instance, some cases of H5N1 infection are characterized by rapid clinical progression, with signs of involvement of the lower respiratory tract, to hospital admission, after which the disease rapidly evolved to the stage in which mechanical ventilation becomes necessary. Patients with severe H5N1 infection develop primary viral pneumonia, early-onset lymphopenia and renal failure within one to two weeks after the onset of symptoms. Elevated transaminase levels have also been detected prior to respiratory deterioration in the majority of patients presenting the severe forms [92].
Economic impact
Economic losses from AI have varied depending on the strain of virus, species of bird infected, number of farms involved, control methods used, and the speed of implementation of control or eradication strategies. Most outbreaks and economic losses have occurred from epidemics of HP or LPAI in commercially raised poultry, predominately chickens and turkeys. Direct losses in HPAI outbreaks have included depopulation and disposal costs, high morbidity and mortality losses, cleaning and disinfection, quarantine and surveillance costs, and indemnities paid for the birds. However, indirect costs such as uncompensated losses to the poultry industry including temporary or permanent loss in poultry exports, income lost by farmers and communities during the production down time, increased consumer costs from reduced supply of poultry products, and losses from decreases in consumer purchases can easily escalate losses by 5-10 folds [94].
The economic costs for eradication of HPAI have varied greatly, but eradication costs have been very high and appear to be proportional to the number of birds that died and were culled [94]. Furthermore, economic losses due to death and culling of domestic poultry, market closures and trade restrictions, have been considerable. The direct and indirect impact of an influenza pandemic would likewise be enormous, affecting the economy as a whole, and in particular health systems, health-care services, political machinery,trade, tourism, biodiversity and essential services such as public transport, education, police and general administration [95].
Four key factors that are identified as contributing to the potential social and economic impact of HPAI includes the zoonotic nature of the disease and the potential for large-scale human deaths, the severe impact of outbreaks on local, and especially vulnerable, populations due to considerable livelihood and production losses, the prolonged financial drain for control costs as the disease becomes endemic, the simultaneous outbreaks across countries and regions as the disease spread rapidly across the continents. If widespread outbreaks persisted without rapid and adequate control measures global production and trade could be severely disrupted. The impact of a single animal outbreak of HPAI on national GDP would depend on the speed with which the disease was controlled, the size and structure of the poultry sector and its relative contribution to GDP. Estimates of global HPAI losses since the start of the outbreaks at the end of 2003 run into billions of dollars [96].
The 2003 and 2004 outbreaks in Asia took Veterinary Services by surprise. As a result the avian Influenza virus was not easily controlled, spread widely, often re-emerged and resulted in the death or destruction of millions of birds. Direct losses were highest in Vietnam (44 million birds, amounting to approximately 17.5% of the poultry population) and Thailand (29 million birds, 14.5% of the poultry population) [96], with long lasting effects on their respective poultry industries due to lost market share.
In regions of the world where there have been HPAI outbreaks, changes in the consumption pattern have been evident, with temporary decreases in poultry consumption. For example, the domestic impact in Turkey in 2006, where 2.5 million birds were culled due to an outbreak of H5N1 HPAI, had a cost of $ 226 million. In the capital city, Ankara, there was a 54% decrease in sales of poultry products, with a 32% decrease in poultry meat prices, and prices of eggs and other poultry products also decreased [84,97,98].
At least 62 countries reported outbreaks of H5N1 HPAI in either domesticated or wild birds between 1996 and 2010 [5]. So HPAI virus has caused devastating economic losses to poultry growers and rural households in Asia, Europe, and Africa [49,99]. In developing countries, most poultry production occurs in small backyard flocks in rural and periurban areas, so outbreaks economically impacted these small farmers more than commercial industries. Between 1996 and 2003, there were 1,645 H5N1 HPAI outbreaks worldwide that resulted in 43 million birds dead or destroyed, and between 2004 and 2007 more than 250 million birds died or were destroyed [96]
Treatment, Prevention and Control
Treatment
There is no effective treatment for avian influenza in poultry. However, good husbandry, proper nutrition, and broad spectrum antibiotics may reduce losses from secondary infections. In human, treatment for avian influenza may vary, depending on the severity of the case. In addition to symptomatic treatment, it can include various drugs, including antibiotics to treat or prevent secondary bacterial pneumonia, and antivirals [100]. Two groups of antiviral drugs such as the adamantanes (amantadine and rimantadine), and neuraminidase inhibitors (zanamivir, oseltamivir, peramivir and laninamivir) are effective against some influenza A viruses, but some of these drugs (peramivir and laninamivir) are not licensed in all countries [101].
The first antiviral drugs described against influenza were the adamantanes, amantadine and rimantadine. These compounds are M2 ion channel blockers that inhibit influenza A replication at the uncoating step (Hayden and Palese, 2002). However, the efficacy of such antiviral drugs is limited by the rapid emergence and transmission of drug-resistance variants [102]. Neuraminidase (NA) inhibitors, such as zanamivir and oseltamivir, were synthesized after the crystal structures of influenza NA complexes with sialic acid and the sialic acid derivative 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid were determined [103]. These inhibitors block the active site of the NA enzyme, inhibiting virus release from infected cells and spread within the respiratory tract [104]. In general, these drugs can potentially offer protection against any Influenza virus that might emerge in humans, as the NA enzymatic active site seems to be highly conserved among all Influenza viruses [105].
Prevention and Control
Disease reporting: A quick response is vital for containing avian influenza outbreaks, and in some cases, for minimizing the risk of zoonotic transmission. In addition to national notification requirements, HPAI viruses and LPAI viruses that contain H5 or H7 must be reported to the OIE by member nations [106]. Veterinarians who encounter or suspect a reportable disease should follow their country-specific guidelines for informing the proper authorities (state or federal veterinary authorities in the U.S. for diseases in animals). Unusual mortality among wild birds should also be reported (e.g., to state, tribal or federal natural resource agencies in the U.S [107].
The control of AI in poultry, from village to commercial sectors, requires farm-to-table risk management. Some of the basic needs include implementation of good agricultural practices such as training of workers in good management and biosecurity practices, in particular poultry cullers,establishing a biosecure environment to isolate poultry from potential AI virus carriers, supplying a source of potable water, providing a feed supply that is secure and free of contaminants, disinfection and decontamination of the premises and equipment prior to the introduction of a new flock or after culling of poultry flocks, establishing routine composting of litter and carcasses for all flocks, and safe disposal of carcasses from known infected farms [108]. During outbreaks, HPAI viruses are normally eradicated by depopulation of infected flocks, combined with other measures such as movement controls, quarantines and perhaps vaccination [109].
Protective measures for zoonotic avian Influenza viruses include controlling the source of the virus (e.g., eradicating HPAI viruses, closing infected poultry markets); avoiding contact with sick animals, animals known to be infected, and their environments; employing good sanitation and hygiene (e.g., hand washing); and using Personal Protective Equipment (PPE) where appropriate [88,110] While the recommended PPE can vary with the situation and risk of illness, it may include respiratory and eye protection such as respirators and goggles, as well as protective clothing including gloves [107,110]. The monitoring of travelers that arrive in a country, with quarantine approaches, the closure of agglomerating places, such as public transportation and schools, could also be necessary actions [35].
Vaccination: vaccination of poultry against avian influenza with inactivated vaccines and live recombinant vaccines (fowl pox H5) has the capacity to increase resistance to infection, to protect poultry from clinical disease and to reduce shedding of virus if vaccinated poultry become infected [111]. So, well-managed vaccination of poultry can reduce the mortality and morbidity rate and the risk to humans by reducing the quantity of circulating virus [112].
In addition to this, annual influenza vaccination is also the best public health intervention to prevent human influenza and available in two trivalent formulations inactivated and live-attenuated that contains an A (H1N1), an A (H3N2), and a B virus strain. A semiannual strain selection process is coordinated by the WHO to determine the composition of the northern and southern hemisphere vaccines [113].
In the field of influenza vaccination, neither commercially available nor experimentally tested vaccines have been shown so far to fulfil all of the requirements [96]. The first aim, which is the protection from clinical disease induced by HPAIV, is achieved by most vaccines. The effectiveness of reduction of virus excretion is important for the main goal of control measures, that is, the eradication of virulent field virus [114]. Therefore, the best strategy to combat a pandemic flu is a rapid and effective vaccine production [115].
Status of the Disease and its Pandemic Threat in Ethiopia
While Ethiopia has not yet experienced an outbreak of Highly Pathogenic Avian Influenza (HPAI), there was an avian flu scare in 2006. This scare caused a significant demand shock that led to a sharp decline in poultry prices [116]. In the same year, a three-year preparedness plan for avian flu, worth US $ 124 million, was approved by the Ethiopian government and international agencies. The avian flu shock was particularly severe in urban areas, where poultry demand decreased by 25-30 percent. As a result of the reduction in urban demand and the subsequent oversupply, poultry prices dropped 50-60 percent, though this plunge was short-lived [117].
Ethiopia is at high risk of the flu pandemic for many reasons. Many birds that possibly carry the virus migrate from affected areas of Europe and Asia to East Africa and reach lakes and wetland found in the rift valley of Ethiopia [118]. That potentially increases the risk of spread into the chicken population. As almost every household in rural areas in Ethiopia practices backyard poultry and humans commonly live with their poultry in the same house or in an attachment where there is no barrier the potential for coming in contact with infected poultry droppings and corpses, which are major sources of infection, is very high. Besides, the uncontrolled animal movements exercised under the prevailing management system are the potential danger of risk of AI in Ethiopia [119].
Legal and illegal trade routes: legal trade of days‐old chicks is carried out by large commercial farms that import day‐old chicks from Egypt, Germany, Holland, Kenya, Saudi Arabia and the United Kingdom. Illegal cross‐border trade results in movement of live poultry from Djibouti and Sudan into Ethiopia. Demand for poultry via this route is high [119]. For this reason, the risk of illegal poultry traders introducing HPAI from an infected zone into Ethiopia should be considered. Generally, because of the importance of the poultry sector (56 millions of poultry), the low level of biosecurity and the relatively high number of migratory water birds wintering in the Rift Valley Lakes, Ethiopia is considered at risk of introduction and spreading for highly pathogenic avian influenza (HPAI) [39,111].
Conclusion and Recommendations
Avian influenza is important veterinary and human health disease around the world. In poultry, it is unusual in that it can cause a range of disease symptoms from a subclinical infection to being highly virulent with high mortality and remains a major health issue for poultry around the world [120-122]. The greatest concern typically has been for highly pathogenic AI because of its severe clinical disease and its effects on trade. However, LPAI also remains a concern because of its ability to cause disease and production losses, it is found more widely than HPAI, and for LPAI H5s and H7s the potential to mutate to HPAI remains ever present. As a result, its outbreaks have caused severe economic losses and agricultural trade restrictions. In addition to this some strains of the viruses have caused sporadic infection of humans, primarily from direct contact with infected birds, producing a high case fatality rate for human infections, but with limited human to human spread and rare transmission through raw food products. Ethiopia is at risk to the outbreak of AI because the country has a lot of wetland areas that are frequently visited by migratory birds from Asia and Europe and because of its illegal cross-border trade. AI viruses are difficult to control because of the wildlife reservoir, the adaptability of the virus, and the lack of good control tools [123-125].
Based on the above concluding remarks the following future directions are forwarded:
✓ Early detection of AI outbreaks and warning system should be designed and implemented.
✓ Timely treatment and annual vaccination should be available.
✓ There should be a public awareness about the health and economic importance of the disease through social and public media.
✓ There should be strong and close collaboration between medical and veterinary professionals to reduce the impact of the disease.
✓ Biosecurity measures should be established to prevent interaction of wild birds and domestic poultry, thereby reducing the risk of AI virus introduction into domestic poultry.
✓ Further research is also needed to develop the most effective vaccines and drugs.
✓ In Ethiopia, national policies should be set on the criteria of importation of poultry and poultry product.
Acknowledgment
First and foremost, all glory should be God and I would like to thank the blessed virgin saint Mary, who prays and save me and illuminates my life.
Next to that, I would like to express my special gratitude to my advisor Mr. Hika Waktole, for his advice, motivation and devotion of time in correcting and reviewing this paper.
I gratefully acknowledge my instructors and friends for their valuable advice and assistance in preparation of this paper. I would not have been able to finish this seminar without their support.
Last but not the least, I would like to thank my family: my parents and to my brothers and sister for supporting me spiritually throughout writing this seminar paper and my life in general.
Host range: Avian Influenza viruses can infect commercial and non-commercial poultry, indoor and outdoor reared poultry, pet birds, wild birds, and a variety of other avian and non avian species [8]. Wild aquatic birds, notably members of the orders Anseriformes (ducks and geese) and Charadriiformes (gulls and shorebirds), are carriers of the full variety of Influenza virus A subtypes, and thus, most probably constitute the natural reservoir of all influenza A viruses [29]. Although aquatic birds are the reservoir for all recognized influenza A subtypes, some viruses have also been detected in other species. However, there appears to be a substantial degree of species specificity. For example, H1N1 and H3N2 have been detected in swine, whereas H3N8 and H7N7 are found in horses [30]. Recently, the horse H3N8 virus jumped the species barrier to cause infections and initiate an evolving outbreak in canines [31].
In addition, while all bird species are thought to be susceptible, some domestic poultry species chickens, turkey, guinea fowl, quail and pheasants are known to be especially vulnerable to the sequelae of infection [32]. For instance, the vast majority of LPAI viruses are maintained in asymptomatic wild birds, particularly birds in wetlands and other aquatic habitats [33]. When these types of viruses from wild birds are transferred to poultry, the viruses may circulate inefficiently and die out; become adapted to the new host and continue to circulate as LPAI viruses; or if they contain H5 or H7, they may evolve into HPAI viruses. In contrast, HPAI viruses are not usually found in wild birds, although they may be isolated transiently near outbreaks in poultry [34].
Geographic distribution: Avian Influenza (AI) virus is a global virus that knows no geographic boundaries, has no political agenda and has been isolated from poultry, captive birds, and wild birds in Africa, Asia, Australia, Europe, and North and South America, and anti-AI antibodies have even been identified in Antarctic penguins [8]. For instance, some noteworthy features of the ongoing outbreaks of infection with highly pathogenic H5N1 in Asia, Europe, Africa, and the Middle East are the number of affected animals and the widening geographic spread [35] (Figure 2). Although human activities may contribute to the geographic spread of H5N1, migratory birds may also play an important role, as is suggested by outbreaks in migratory birds in remote areas such as Mongolia [36]. LPAI viruses are cosmopolitan in wild birds, although the specific viruses differ between regions [37]. Annual outbreaks of influenza occur regularly in temperate regions of the world with remarkable seasonality, defined by peak incidence in the colder months of the year. Such annual outbreaks vary in severity [38].
The occurrence of AI epidemic in Africa is due to the flyways of migratory birds, which link the endemic and newly infected countries with free areas on this continent, and because of the risk of introduction through legal and illegal trade [39]. From the sub-Saharan countries, Ethiopia and Kenya have not yet experienced any outbreaks, but the virus has been circulating in neighboring countries, e.g. Sudan, and it could enter these countries through various pathways, including illegal bird trade. However, Ghana and Nigeria have both experienced several outbreaks and are on the same bird flyways [40].
Source of infection: Aquatic wild birds are the natural reservoir of influenza A viruses and are thought to be the principal source of viral spread to other species [30]. The virus replication is primarily limited to the epithelial cells of the intestinal tract [41,42], and infected birds remain asymptomatic but shed the virus into the environment via feces, and less frequently saliva and nasal secretions [43] (Figure 3). Sources of infection may also include other animals like swine [44].
Mode of transmission between birds: As the natural reservoir for AI, infected waterfowl are believed to pass the AI virus to domestic poultry by the fecal-oral route through contaminated water sources, feed, and housing facilities/ shared environment, where infectious virus can be excreted by birds for up to 10 days. The virus can survive at 4oC in feces for at least 35 days [45,46]. Transmission of influenza A viruses from aquatic birds likely occurs through shared water source and infected migratory birds may result in wide geographic spread of viruses. Although flyways of bird populations are partially separated, allowing distinct gene pools of viruses to develop [30], evidence suggests that there is also interregional transmission by infected migratory birds. One study has demonstrated that H2 influenza A viruses isolated from shorebirds in North America between 1985 and 1998 contain genes belonging to a Eurasian lineage of H2 viruses [47].
Transmission is also possible via aerosols or airborne droplets, as well as by wind dispersion of fomites including contaminated dust. Indirect exposures occur through surface contamination of equipment, clothing, and shoes from dust, feces, secretions, and contaminated feathers [48]. Interspecies transmissions of AI viruses have also occurred in other species as well. [49].
Pathogenicity and pathogenesis
Avian Influenza viruses are classified as either low pathogenic (also called low pathogenicity) avian Influenza viruses or highly pathogenic (high pathogenicity) avian Influenza viruses and defined as HPAI or LPAI by its ability to cause severe disease in intravenously inoculated young chickens in the laboratory, or by its possession of certain genetic features that have been associated with high virulence in HPAI viruses (i.e., the sequence at the HA cleavage site) [50,51]. HPAI viruses usually cause severe disease in chicken and turkey flocks, while LPAI infections are generally much milder in all avian species [52].
Pathogenicity as a general viral property in influenza A viruses is a polygenic trait and depends largely on an optimal gene constellation affecting host and tissue tropism, replication efficacy and immune evasion mechanisms amongst others. In addition, host and species-specific factors contribute to the outcome of infection, which, after interspecies transmission, is therefore unpredictable [53]. LPAI viruses can be introduced by various pathways into poultry flocks. Following a variable and indecisive period of circulation (and, presumably, adaptation) in susceptible poultry populations, these viruses can saltatorily mutate into the highly pathogenic form [54].
First, in poultry, the process of pathogenesis begins by inhalation or ingestion of infectious LP or HPAI virions. Because trypsin-like enzymes in respiratory and intestinal epithelial cells allow cleavage of the surface hemagglutinin, multiple replication cycles occur in respiratory and/or intestinal tracts with release of infectious virions. Second, with HPAI viruses, after initial replication in respiratory epithelium, the virions invade the submucosa, entering capillaries. The virus replicates within endothelial cells and spreads via the vascular or lymphatic systems to infect and replicate in a variety of cell types in visceral organs, brain, and skin. Alternatively, the virus may become systemic before having extensive replication in vascular endothelial cells. The virus is present in the plasma, red and white blood cell fractions. Macrophages appear to play a role in systemic virus spread. The presence of a hemagglutinin proteolytic cleavage site that can be cut by ubiquitous furin like cellular enzymes is responsible for this pantropic replication. Clinical signs and death are due to multiple organ failure [8].
Third, for the LPAI viruses, replication usually is limited to the respiratory or intestinal tracts. Illness or death is most often from respiratory damage, especially if accompanied by secondary bacterial infections. Sporadically in some species, the LPAI viruses spread systemically, replicating and causing damage in kidney tubules, pancreatic acinar epithelium, oviduct and other organs with epithelial cells having trypsin-like enzymes. Pathogenesis of the infection process is less well understood in non-gallinaceous birds [8].
Clinical manifestation and pathological lesions in poultry
The incubation period of avian Influenza viruses in poultry can be a few hours to a few days in individual birds, and up to 2 weeks in the flock [33]. For mammals, the period is also thought to be short, and might be as little as 1-2 days in some cases [55]. Following an incubation period of usually a few days depending upon the characteristics of the isolate, the dose of inoculum, the species, and age of the bird, the clinical presentation of avian influenza in birds is variable and symptoms are fairly unspecific [56].
The symptoms following infection with low pathogenic AIV may be as discrete as ruffled feathers, transient reductions in egg production or weight loss combined with a slight respiratory disease [57]. In its highly pathogenic form, the illness in chickens and turkeys is characterised by a sudden onset of severe symptoms and a mortality that can approach 100% within 48 hours [53]. Often, only a section of a stable is affected. Many birds die without premonitory signs so that sometimes poisoning is suspected in the beginning [58].
Oedema, visible at feather-free parts of the head, cyanosis of comb, wattles and legs, greenish diarrhoea and laboured breathing may also be inconsistently present. In layers, soft-shelled eggs are seen initially, but any laying activities cease rapidly with progression of the disease [56]. Nervous symptoms including tremor, unusual postures (torticollis), and problems with co-ordination (ataxia) also dominate the picture in less vulnerable species such as ducks, geese, and ratites [59]. Microbiological features found in infected poultry are infarction of tissue and inflammation of inner organs which are generally found in brain, heart, lungs, pancreas, primary and secondary lymphoid organs [51].
Diagnosis
Clinical diagnosis: Clinically the disease is indistinguishable because lesions and symptoms are to variable and confuse with other diseases and AI virus cannot be diagnosed by clinical signs and symptoms alone. Therefore, confirmation should be undertaken by specialized laboratories, serology and virology are necessary [60].
Laboratory diagnosis: Avian Influenza viruses can be detected in oropharyngeal, tracheal and/or cloacal swabs from live birds, with differing recovery rates from each site depending on the virus, species of bird and other factors. Very small swabs can be valuable in small birds, but feces can be substituted if cloacal samples are not practical (e.g., can not be collected without harming the bird) [18]. Immature feathers may also be a useful sample (Nuradji et al., 2015). Samples from internal organs are also tested in dead birds suspected of having HPAI [50].
Virus isolation: virus isolation by inoculating the sample into hatching chicken eggs for detecting a property of red blood cells precipitation can be performed in all species, and can be useful for virus characterization [61] This technique is the “gold standard” but laborious and time insensitive, used primarily for diagnosis of first clinical case and to obtain virus isolated for further laboratory analysis [62]. laboratory diagnosis of AI viruses can also be performed by serological test such as hemagglutinin inhibition test, Agar Gel Immune Diffusion (AGID), antigen-detection ELISAs or other immunoassays, or by a molecular test such as RT-PCR [30]. The viruses can be identified as influenza A viruses with hemagglutination inhibition test, in which the Hemagglutinin (HA) protein of avian influenza has the property to agglutinate erythrocytes from a number of species including horses. A specific antibody to the antigenic sites on the avian influenza HA molecule prevents or inhibits the hemagglutination reaction. Therefore, hemagglutination inhibition test can be used to type the patient antibodies to avian Influenza virus when standard avian influenza antigen is available as reference material [61].
Use of the AGID test to demonstrate nucleocapsid or matrix antigens is also a satisfactory way to indicate the presence of influenza A virus in amnioallantoic and chorioallantoic fluid, but various experimental and commercial rapid, solid-phase antigen-capture ELISAs (AC-ELISAs) are an effective alternative [63]. They use a monoclonal antibody against the nucleoprotein; they should be able to detect any influenza A virus. The main advantage of these tests is that they can demonstrate the presence of influenza A within 15 minutes. The disadvantages are that they may lack sensitivity, they may not have been validated for different species of birds, subtype identification is not achieved and the kits are expensive [64].
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) is another powerful technique for the identification of Influenza virus genomes and allow for sensitive and specific detection of viral nucleic acid [24]. RT-PCR techniques on clinical specimens can, with the correctly defined primers, result in rapid detection and subtype identification (at least of H5 and H7), including a DNA product that can be used for nucleotide sequencing [65]. However, the preferred molecular detection tests for influenza A virus is the real-time RT-PCR, a modification to the RT-PCR that reduces the time for both identification of virus subtype and sequencing [66]. A disadvantage of RT PCR methods is its proneness for contamination and the consequent risk of false positive results [24].
Differential diagnosis: Diseases must be considered in the differential diagnosis of HPAI because of their ability to cause a sudden onset of disease accompanied by high mortality or haemostasis in wattles and combs are velogenic Newcastle disease, infectious laryngotracheitis (chickens), duck plague, acute poisonings, acute fowl cholera (pasteurellosis) and other septicaemic diseases, bacterial cellulitis of the comb and wattles [56]. For LPAI viruses, other causes of respiratory disease and drops in egg production must be investigated such as lentogenic Newcastle disease virus, avian pneumovirus and other paramyxoviruses, infectious laryngotracheitis, infectious bronchitis, chlamydia, mycoplasma, and various bacteria [9].
Impact 0f the Disease
Public health impact
Outbreaks of HPAI viruses in wild and domestic birds are rare, but once infection occurs it can be serious from veterinary, medical, and public health perspectives [67]. The two most commonly reported avian Influenza viruses from human clinical cases have been the Asian lineage H5N1 HPAI viruses, and recently, H7N9 LPAI viruses in China [68,69]. There are currently no reported human infections caused by Asian lineage H5N8 viruses, although four infections with H5N6 viruses have been detected in China since 2014 [70,71].
Illnesses caused by other subtypes have also been reported sporadically, with documented clinical cases caused by H9N2 (Eurasian lineage), H6N1 and multiple H7 and H10 avian Influenza viruses [72,73]. Whether these infections are truly less common than subtypes such as H5N1 is unclear: viruses that tend to cause milder illnesses (e.g., H9N2 viruses) are less likely to be identified than those causing severe disease. Serological surveys in some highly exposed populations suggest the possibility of low level exposure to HA types found in birds, including H4, H5, H6, H7, H9, H10, H11 and H12 [74]. Adaptation to humans is possible, though rare, and some previous human pandemics were caused by partially or wholly avian viruses [75,76].
In general, Influenza viruses exhibit host species adaptation with transmission occurring most frequently and with ease between individuals of the same species; occasionally interspecies transmission to closely related species occurs [77]. On rare occasions, AI viruses have exhibited interspecies transmissibility to humans [9]. Although rare, AI viruses or their genes have been transferred to humans: transfer of complete AI viruses with individual sporadic infections, and appearance of individual AI viral gene segments in pandemic human Influenza viruses (i.e., reassortment of gene segments) [8].
Human pandemic: Over the past 150 years at least four pandemics of avian influenza occurred at irregular intervals, including three in the 20th century. These have caused high attack rates in all susceptible age groups with high morbidity and mortality. Earliest pandemic occurred in 1918-19 and is widely known as "Spanish flu.” It was caused by (H1N1) and lead to the highest number of known flu deaths; more than 500,000 people died in the United States alone and 20 to 50 million may have died worldwide. Second was "Asian flu" which occurred in 1957-58. Its causative agent was (H2N2). It resulted in about 70,000 deaths in the United States. Third pandemic commonly called as "Hong Kong flu" was caused by (H3N2) in 1968-69. There were approximately 34,000 deaths in the United States. This virus was first detected in Hong Kong in early 1968 [78].
Since the outbreaks of H5N1 HPAI in poultry and humans in Hong Kong in 1997 and H7N7HPAI in the Netherlands in 2003, there have been concerns that AI viruses could persist in some poultry populations and emerge as a pandemic virus for humans through multiple mutations or reassortment [67]. The 2009 pandemic H1N1 was the result of a reintroduction of H1N1, which over time had mutated and reassorted influenza A genes from various avian, human, and swine Influenza viruses [79,80,81]. Human infection with avian influenza A (H7N9) was also first reported in China in March 2013, and since then hundreds of human cases have been confirmed. Today, AI viruses of concern as potential pandemic strains are some H5, H7, and H9 subtypes that have crossed the human species barrier multiple times to produce sporadic infections [82].
The current spread of avian influenza H5N1 in domestic poultry flocks and wild birds across the world, as well as the demonstrated ability of this virus to cross the species barrier and infect humans, has lead to a high level of concern that a pandemic may develop. For a pandemic to arise, three pre-requisites have been identified such as a new virus subtype to which the population has little or no immunity must emerge, the new virus must be able to replicate in humans and cause serious illness, and the new virus must be efficiently transmitted from one human to another [83].
Transmission to humans: The close proximity of birds to humans increases the risk of transmission to humans via aerosol or large airborne droplets, fecal contamination with dispersion via fomites, and direct contact with infected birds. Direct contact with infected poultry, or surfaces and objects contaminated by their faeces, is presently considered the main route of human infections. As infected birds shed large quantities of virus in their faces, opportunities for exposure to infected droppings or to environments contaminated by the virus are abundant under such conditions. Also, sick birds may be slaughtered for consumption in the developing world, leading to increased risk of exposure [84]. This is because of many households in developing countries depends on poultry for income and food, many families sell or slaughter them or even consume them when signs of illness appear in a flock [85].
In general, people at greatest risk for AI virus exposure and infection include farm workers, live bird market workers, butchers and home processors of poultry, hunters that slaughter, eviscerater, and defeather infected wild birds, and those preparing to cook contaminated meat getting viruses on their hands and transferring them by touching mucus membranes [86]. However, properly cooked and properly handled poultry and poultry products can safely be consumed [85].
H5N1 HPAI virus has expanded its host range, as it has infected dogs and other mammals through the consumption of uncooked infected poultry, wild birds, or their products [67,86]. This has raised concern that dogs and other pets also have the potential to be intermediate carriers that can transfer the H5N1 Influenza virus to humans. Although rare, evidence of direct human to human transmission of H5N1 associated with a poultry outbreak also occurred in Southeast Asia. Sustained human to human transmissibility of H5N1 HPAI would require genetic adaption of AI PB2 internal protein [87].
Clinical sign in humans: Most zoonotic infections caused by Asian lineage H5N1 HPAI viruses seem to become apparent within approximately 5 days, although the incubation period for some cases may be as long as 8 and possibly 17 days [88,89]. Estimates of the mean incubation period for the zoonotic H7N9 viruses have varied from 3 days (in two analyses, which considered large numbers of cases) to 5-6 days, with a range of 1-13 days [90,91].
Clinical symptoms of avian influenza infections in humans range from asymptomatic infection or mild conjunctivitis to fatal systemic disease and multiorgan failure including severe or fatal respiratory, gastrointestinal, or neurological syndromes [24,2]. Initial symptoms include headache, fever, fatigue, myalgia, odynophagia, cough and rhinorrhea, abdominal pain, vomiting, diarrhea, hepatic dysfunction, Reye's syndrome, pancytopenia, renal failure, pulmonary hemorrhage, acute respiratory distress syndrome and septic shock have been reported with varying frequency [92,93].
For instance, some cases of H5N1 infection are characterized by rapid clinical progression, with signs of involvement of the lower respiratory tract, to hospital admission, after which the disease rapidly evolved to the stage in which mechanical ventilation becomes necessary. Patients with severe H5N1 infection develop primary viral pneumonia, early-onset lymphopenia and renal failure within one to two weeks after the onset of symptoms. Elevated transaminase levels have also been detected prior to respiratory deterioration in the majority of patients presenting the severe forms [92].
Economic impact
Economic losses from AI have varied depending on the strain of virus, species of bird infected, number of farms involved, control methods used, and the speed of implementation of control or eradication strategies. Most outbreaks and economic losses have occurred from epidemics of HP or LPAI in commercially raised poultry, predominately chickens and turkeys. Direct losses in HPAI outbreaks have included depopulation and disposal costs, high morbidity and mortality losses, cleaning and disinfection, quarantine and surveillance costs, and indemnities paid for the birds. However, indirect costs such as uncompensated losses to the poultry industry including temporary or permanent loss in poultry exports, income lost by farmers and communities during the production down time, increased consumer costs from reduced supply of poultry products, and losses from decreases in consumer purchases can easily escalate losses by 5-10 folds [94].
The economic costs for eradication of HPAI have varied greatly, but eradication costs have been very high and appear to be proportional to the number of birds that died and were culled [94]. Furthermore, economic losses due to death and culling of domestic poultry, market closures and trade restrictions, have been considerable. The direct and indirect impact of an influenza pandemic would likewise be enormous, affecting the economy as a whole, and in particular health systems, health-care services, political machinery,trade, tourism, biodiversity and essential services such as public transport, education, police and general administration [95].
Four key factors that are identified as contributing to the potential social and economic impact of HPAI includes the zoonotic nature of the disease and the potential for large-scale human deaths, the severe impact of outbreaks on local, and especially vulnerable, populations due to considerable livelihood and production losses, the prolonged financial drain for control costs as the disease becomes endemic, the simultaneous outbreaks across countries and regions as the disease spread rapidly across the continents. If widespread outbreaks persisted without rapid and adequate control measures global production and trade could be severely disrupted. The impact of a single animal outbreak of HPAI on national GDP would depend on the speed with which the disease was controlled, the size and structure of the poultry sector and its relative contribution to GDP. Estimates of global HPAI losses since the start of the outbreaks at the end of 2003 run into billions of dollars [96].
The 2003 and 2004 outbreaks in Asia took Veterinary Services by surprise. As a result the avian Influenza virus was not easily controlled, spread widely, often re-emerged and resulted in the death or destruction of millions of birds. Direct losses were highest in Vietnam (44 million birds, amounting to approximately 17.5% of the poultry population) and Thailand (29 million birds, 14.5% of the poultry population) [96], with long lasting effects on their respective poultry industries due to lost market share.
In regions of the world where there have been HPAI outbreaks, changes in the consumption pattern have been evident, with temporary decreases in poultry consumption. For example, the domestic impact in Turkey in 2006, where 2.5 million birds were culled due to an outbreak of H5N1 HPAI, had a cost of $ 226 million. In the capital city, Ankara, there was a 54% decrease in sales of poultry products, with a 32% decrease in poultry meat prices, and prices of eggs and other poultry products also decreased [84,97,98].
At least 62 countries reported outbreaks of H5N1 HPAI in either domesticated or wild birds between 1996 and 2010 [5]. So HPAI virus has caused devastating economic losses to poultry growers and rural households in Asia, Europe, and Africa [49,99]. In developing countries, most poultry production occurs in small backyard flocks in rural and periurban areas, so outbreaks economically impacted these small farmers more than commercial industries. Between 1996 and 2003, there were 1,645 H5N1 HPAI outbreaks worldwide that resulted in 43 million birds dead or destroyed, and between 2004 and 2007 more than 250 million birds died or were destroyed [96]
Treatment, Prevention and Control
Treatment
There is no effective treatment for avian influenza in poultry. However, good husbandry, proper nutrition, and broad spectrum antibiotics may reduce losses from secondary infections. In human, treatment for avian influenza may vary, depending on the severity of the case. In addition to symptomatic treatment, it can include various drugs, including antibiotics to treat or prevent secondary bacterial pneumonia, and antivirals [100]. Two groups of antiviral drugs such as the adamantanes (amantadine and rimantadine), and neuraminidase inhibitors (zanamivir, oseltamivir, peramivir and laninamivir) are effective against some influenza A viruses, but some of these drugs (peramivir and laninamivir) are not licensed in all countries [101].
The first antiviral drugs described against influenza were the adamantanes, amantadine and rimantadine. These compounds are M2 ion channel blockers that inhibit influenza A replication at the uncoating step (Hayden and Palese, 2002). However, the efficacy of such antiviral drugs is limited by the rapid emergence and transmission of drug-resistance variants [102]. Neuraminidase (NA) inhibitors, such as zanamivir and oseltamivir, were synthesized after the crystal structures of influenza NA complexes with sialic acid and the sialic acid derivative 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid were determined [103]. These inhibitors block the active site of the NA enzyme, inhibiting virus release from infected cells and spread within the respiratory tract [104]. In general, these drugs can potentially offer protection against any Influenza virus that might emerge in humans, as the NA enzymatic active site seems to be highly conserved among all Influenza viruses [105].
Prevention and Control
Disease reporting: A quick response is vital for containing avian influenza outbreaks, and in some cases, for minimizing the risk of zoonotic transmission. In addition to national notification requirements, HPAI viruses and LPAI viruses that contain H5 or H7 must be reported to the OIE by member nations [106]. Veterinarians who encounter or suspect a reportable disease should follow their country-specific guidelines for informing the proper authorities (state or federal veterinary authorities in the U.S. for diseases in animals). Unusual mortality among wild birds should also be reported (e.g., to state, tribal or federal natural resource agencies in the U.S [107].
The control of AI in poultry, from village to commercial sectors, requires farm-to-table risk management. Some of the basic needs include implementation of good agricultural practices such as training of workers in good management and biosecurity practices, in particular poultry cullers,establishing a biosecure environment to isolate poultry from potential AI virus carriers, supplying a source of potable water, providing a feed supply that is secure and free of contaminants, disinfection and decontamination of the premises and equipment prior to the introduction of a new flock or after culling of poultry flocks, establishing routine composting of litter and carcasses for all flocks, and safe disposal of carcasses from known infected farms [108]. During outbreaks, HPAI viruses are normally eradicated by depopulation of infected flocks, combined with other measures such as movement controls, quarantines and perhaps vaccination [109].
Protective measures for zoonotic avian Influenza viruses include controlling the source of the virus (e.g., eradicating HPAI viruses, closing infected poultry markets); avoiding contact with sick animals, animals known to be infected, and their environments; employing good sanitation and hygiene (e.g., hand washing); and using Personal Protective Equipment (PPE) where appropriate [88,110] While the recommended PPE can vary with the situation and risk of illness, it may include respiratory and eye protection such as respirators and goggles, as well as protective clothing including gloves [107,110]. The monitoring of travelers that arrive in a country, with quarantine approaches, the closure of agglomerating places, such as public transportation and schools, could also be necessary actions [35].
Vaccination: vaccination of poultry against avian influenza with inactivated vaccines and live recombinant vaccines (fowl pox H5) has the capacity to increase resistance to infection, to protect poultry from clinical disease and to reduce shedding of virus if vaccinated poultry become infected [111]. So, well-managed vaccination of poultry can reduce the mortality and morbidity rate and the risk to humans by reducing the quantity of circulating virus [112].
In addition to this, annual influenza vaccination is also the best public health intervention to prevent human influenza and available in two trivalent formulations inactivated and live-attenuated that contains an A (H1N1), an A (H3N2), and a B virus strain. A semiannual strain selection process is coordinated by the WHO to determine the composition of the northern and southern hemisphere vaccines [113].
In the field of influenza vaccination, neither commercially available nor experimentally tested vaccines have been shown so far to fulfil all of the requirements [96]. The first aim, which is the protection from clinical disease induced by HPAIV, is achieved by most vaccines. The effectiveness of reduction of virus excretion is important for the main goal of control measures, that is, the eradication of virulent field virus [114]. Therefore, the best strategy to combat a pandemic flu is a rapid and effective vaccine production [115].
Status of the Disease and its Pandemic Threat in Ethiopia
While Ethiopia has not yet experienced an outbreak of Highly Pathogenic Avian Influenza (HPAI), there was an avian flu scare in 2006. This scare caused a significant demand shock that led to a sharp decline in poultry prices [116]. In the same year, a three-year preparedness plan for avian flu, worth US $ 124 million, was approved by the Ethiopian government and international agencies. The avian flu shock was particularly severe in urban areas, where poultry demand decreased by 25-30 percent. As a result of the reduction in urban demand and the subsequent oversupply, poultry prices dropped 50-60 percent, though this plunge was short-lived [117].
Ethiopia is at high risk of the flu pandemic for many reasons. Many birds that possibly carry the virus migrate from affected areas of Europe and Asia to East Africa and reach lakes and wetland found in the rift valley of Ethiopia [118]. That potentially increases the risk of spread into the chicken population. As almost every household in rural areas in Ethiopia practices backyard poultry and humans commonly live with their poultry in the same house or in an attachment where there is no barrier the potential for coming in contact with infected poultry droppings and corpses, which are major sources of infection, is very high. Besides, the uncontrolled animal movements exercised under the prevailing management system are the potential danger of risk of AI in Ethiopia [119].
Legal and illegal trade routes: legal trade of days‐old chicks is carried out by large commercial farms that import day‐old chicks from Egypt, Germany, Holland, Kenya, Saudi Arabia and the United Kingdom. Illegal cross‐border trade results in movement of live poultry from Djibouti and Sudan into Ethiopia. Demand for poultry via this route is high [119]. For this reason, the risk of illegal poultry traders introducing HPAI from an infected zone into Ethiopia should be considered. Generally, because of the importance of the poultry sector (56 millions of poultry), the low level of biosecurity and the relatively high number of migratory water birds wintering in the Rift Valley Lakes, Ethiopia is considered at risk of introduction and spreading for highly pathogenic avian influenza (HPAI) [39,111].
Conclusion and Recommendations
Avian influenza is important veterinary and human health disease around the world. In poultry, it is unusual in that it can cause a range of disease symptoms from a subclinical infection to being highly virulent with high mortality and remains a major health issue for poultry around the world [120-122]. The greatest concern typically has been for highly pathogenic AI because of its severe clinical disease and its effects on trade. However, LPAI also remains a concern because of its ability to cause disease and production losses, it is found more widely than HPAI, and for LPAI H5s and H7s the potential to mutate to HPAI remains ever present. As a result, its outbreaks have caused severe economic losses and agricultural trade restrictions. In addition to this some strains of the viruses have caused sporadic infection of humans, primarily from direct contact with infected birds, producing a high case fatality rate for human infections, but with limited human to human spread and rare transmission through raw food products. Ethiopia is at risk to the outbreak of AI because the country has a lot of wetland areas that are frequently visited by migratory birds from Asia and Europe and because of its illegal cross-border trade. AI viruses are difficult to control because of the wildlife reservoir, the adaptability of the virus, and the lack of good control tools [123-125].
Based on the above concluding remarks the following future directions are forwarded:
✓ Early detection of AI outbreaks and warning system should be designed and implemented.
✓ Timely treatment and annual vaccination should be available.
✓ There should be a public awareness about the health and economic importance of the disease through social and public media.
✓ There should be strong and close collaboration between medical and veterinary professionals to reduce the impact of the disease.
✓ Biosecurity measures should be established to prevent interaction of wild birds and domestic poultry, thereby reducing the risk of AI virus introduction into domestic poultry.
✓ Further research is also needed to develop the most effective vaccines and drugs.
✓ In Ethiopia, national policies should be set on the criteria of importation of poultry and poultry product.
Acknowledgment
First and foremost, all glory should be God and I would like to thank the blessed virgin saint Mary, who prays and save me and illuminates my life.
Next to that, I would like to express my special gratitude to my advisor Mr. Hika Waktole, for his advice, motivation and devotion of time in correcting and reviewing this paper.
I gratefully acknowledge my instructors and friends for their valuable advice and assistance in preparation of this paper. I would not have been able to finish this seminar without their support.
Last but not the least, I would like to thank my family: my parents and to my brothers and sister for supporting me spiritually throughout writing this seminar paper and my life in general.
- Ensimnger ME. poultry science. 3rd ed. Danville IL. editor. Interstate publishers. INC, USA.1992; pp. 2-13.
- Abdel-Ghafar A, Chotpitayasunondh T, Gao Z, Hayden F, Nguyen D, de Jong M, etal. Update on avian influenza A (H5N1) virus infection in humans. New Engl J Med. 2008; 358: 261-273. DOI: 10.1056/NEJMra0707279
- Alexander D. A review of avian influenza in different bird species. Vet Microbiol. 2000; 74: 3-13. DOI: 10.1016/s0378-1135(00)00160-7
- Lee CW, Saif YM. Avian Influenza virus. Comp. Immunol. Microbiol. Infect Dis. 2009; 32: 301-310. DOI: 10.1016/j.cimid.2008.01.007
- OIE. OIE Terr. Anim. Health Code: Avian influenza, Article 10.4.1. In Health Standards. Paris, Fr: OIE. 2009.
- Alexander D. An overview of the epidemiology of avian influenza. Vaccine. 2006; 25: 5637-5644. DOI: 10.1016/j.vaccine.2006.10.051
- WHO. Avian influenza: assessing the pandemic threat. WHO, Geneva Switzerland. 2005.
- Swayne DE, Halvorson DA Influenza. In: Diseases of Poultry. 12th ed. Saif YM, Glisson JR, Fadly AM, McDougald LR, Nolan L. Editors. Ames, Iowa, USA: Lowa State University Press and Wiley-Blackwell Publishing; 2008. pp. 153-184.
- Easterday BC, Hinshaw VS, Halvorson DA. Influenza. In: Diseases of Poultry, 10th ed. Calnek BW, Barnes H J, Beard CW, McDougald LR, Saif YM. Iowa State University Press, Ames, Iowa. 1997. pp. 583-605.
- Hayden F, Klimov A, Tashiro M, Ha Y, Monto A, McKimm Breschkin J, et al. Neuraminidase inhibitor susceptibility network position statement: antiviral resistance in influenza A/H5N1 viruses. Antivir. Ther. 2005; 10: 873-877. https://bit.ly/2X3sjSB
- Hayden FG. Antiviral resistance in Influenza viruses: Implications for management and pandemic response. N. Engl. J. Med. 2006; 354: 785-788. https://bit.ly/339xvbc
- Wang QP, Chen XG, Lun ZR. Possible Avian Influenza (H5N1) from Migratory bird. Emerg Infec Dis . 2007; 13: 1120-1121
- Webster RG, Peiris M, Chen H, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg Infec Dis. 2006; 12: 3-8. DOI: 10.3201/eid1201.051024
- Suarez DL, Schultz CS. Immunology of avian Influenza virus: A review. Dev Comp Immunol. 2000; 24: 269-283. DOI: 10.1016/s0145-305x(99)00078-6
- Alexander DJ, Brown IH. History of high pathogenic avian influenza. Rev Sci Tech. 2009; 28: 19-38. DOI: 10.20506/rst.28.1.1856
- WHO: H5N1 avian influenza: Timeline of major events. 2012. https://bit.ly/3gnKPwN
- a. Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013; 9: 1003657. DOI: 10.1371/journal.ppat.1003657
- OIE. Manual of diagnostic tests and vaccines for terrestrial animals. Paris; OIE. Avian influenza. 2015.
- Lamb RA, Krug RM. Orthomyxoviridae. The viruses and their replication. In: Knipe DM, Howley PM, Griffin, DE : Fields Virology, 4th ed. Philadelphia, PA, USA: Lippincott Williams and Wilkins. 2001; pp. 1487-1531.
- Noda T, Sagara H, Yen A, Takada A, Kida H, Cheng RH, et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature. 2006; 439: 490-492. DOI: 10.1038/nature04378
- Takeda M, Leser G, Russell C, Lamb R. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc Natl Acad Sci USA. 2003; 100: 14610-14617. DOI: 10.1073/pnas.2235620100
- Kido H, Murakami M, Oba K, Chen Y, Towatari T. Cellular proteinases trigger the infectivity of the influenza A and Sendai viruses. Mol Cells. 1999; 9: 235-244. https://bit.ly/3f5g1zm
- Li Z, Jiang Y, Jiao P, Wang A, Zhao F, Tian G,et al. The NS1 gene contributes to the virulence of h5n1 avian Influenza viruses. J Virol. 2006; 80: 11115-11123. DOI: 10.1128/JVI.00993-06
- De Jong MD, Hien TT. Avian influenza A (H5N1). J. Clin. Virol. 2006; 35: 2-13. DOI: 10.1016/j.jcv.2005.09.002
- Fenner F, Bachmann P, Gibbs E, Murphy F, Studdert M, White D.Veterinary virology. 3rd ed San Diego, CA: Academic Press Inc. Orthomyxoviridae. 1987; pp. 473-484. https://bit.ly/3hDck5s
- Escorcia M, Vazquez L, Mendez S, Rodrıguez R, Lucio E, Nava M. Avian influenza: Genetic evolution under vaccination pressure. Virol. 2008; 5: 1-5. https://bit.ly/2Dg3qw4
- Fergusso NM, Galvani AP, Bush RM. Ecological and immunological determinants of influenza evolution. Nature. 2003; 422: 428-433. https://go.nature.com/3g9LKAv
- Webster RG, Hulse DJ. Microbial adaptation and change: avian influenza. Rev Sci Tech. 2004; 23: 453-465. DOI: 10.20506/rst.23.2.1493
- Widjaja L, Krauss S, Webby RJ, Xie T, Webster RG. Matrix gene of influenza A viruses isolated from wild aquatic birds: Ecology and emergence of influenza a viruses. J Virol. 2004; 78: 8771-8779. DOI: 10.1128/JVI.78.16.8771-8779.2004
- Webster RG, Bean WJ, Gorman OT, Chambers T, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56: 152-179. PubMed: https://pubmed.ncbi.nlm.nih.gov/1579108/
- Crawford P, Dubovi E, Castleman W, Stephenson I, Gibbs E, Chen L, et al. Transmission of equine Influenza virus to dogs. Sci. 2005; 310: 482-485. DOI: 10.1126/science.1117950
- Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 Influenza virus polymerase genes. Nature. 2005; 437: 889-893. DOI: 10.1038/nature04230
- Swayne DE. Overview of avian influenza. In: Aiello SE, Moses MA. Editors. The Merck veterinary manual. Whitehouse Station, NJ: Merck and Co; 2014.
- Stallknecht D, Nagy E, Hunter D, Slemons R. Avian influenza. In: Thomas N, Hunter D, Atkinson C. editors. Infectious Diseases of Wild Birds. Ames, IA: Blackwell Publishing; 2007. pp. 108-130. https://bit.ly/2X8CIfI
- WHO. Epidemic and Pandemic Alert a Response. Ref Survey Quart. 2006; 25: 100-103.
- Normile D. Are wild birds to blame? Sci. 2005; 310: 426-428. DOI: 10.1126/science.310.5747.426
- Gonzalez-Reiche AS, Perez DR. Where do avian Influenza viruses meet in the Americas? Avian Dis. 2012; 56: 1025-1033. DOI: 10.1637/10203-041412-Reg.1
- Acha P, Szyfres B. Zoonoses and communicable diseases common to man and animals. Chlamydiosis, rickettsioses and viruses. 3rd ed. Washington DC, (Pan American Health Organization (PAHO). Scientific and Technical Publication No. 580. Influenza. 2003; 2: 155-172. https://bit.ly/2P7MaeN
- Goutard F, Roger F, Guitian J, Balança G, Argaw K, Demissie A, et al. Conceptual framework for AI risk assessment in Africa: The case of Ethiopia. Avian Dis. 2007; 51: 504-506.
- Henning J, Bett B, Okike I, Abdu P, Perry B. Incidence of highly pathogenic avian influenza H5N1 in Nigeria, 2005-2008. Transbound. Emerg Dis. 2013; 60: 222-30. https://bit.ly/3069AHM
- Ito T, Suzuki Y, Suzuki T, Takada A, Horimoto T, Wells K, et al. Recognition of N-glycolylneuraminic acidlinked to galactose by the alpha 2,3 linkage is associated with intestinal replication of influenza A virus inducks. J Virol. 2000; 74: 9300-9305. DOI: 10.1128/jvi.74.19.9300-9305.2000
- Webster RG, Krauss S, Hulse P, Sturm R. Evolution of influenza A virus in wild birds. J Wildl Dis. 2007; 43: 1-6. https://bit.ly/2X93Vil
- Hinshaw VS. The nature of avian influenza in migratory waterfowl, including interspecies transmission: In 1986 Proceedings of the Second International Symposium on Avian Influenza, Athens, GA: Am Assoc. Avian Pathol. 1985; pp. 133-141.
- Philippa JDW, Munster VJ, Bolhuis HV, Bestebroer TM, Schaftenaar W, Beyer WE, et al. Highly pathogenic avian influenza (H7N7): Vaccination of zoo birds and transmission to nonpoultry species. Vaccine. 2005; 23: 5743-5750. DOI: 10.1016/j.vaccine.2005.09.013
- Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. Persistence of H5 and H7 avian influenzaviruses in water. Avian Dis. 2007; 51: 285-289. DOI: 10.1637/7636-042806R.1
- Stallknecht DE, Brown JD. Wild birds and the epidemiology of avian influenza. J Wildl Dis. 2007; 43: 15-20.
- Makarova N, Kaverin N, Krauss S, Senne D, Webster R. Transmission of Eurasian avian H2 Influenza virus to shorebirds in North America. J Gen Virol. 1999; 80: 3167-3171. DOI: 10.1099/0022-1317-80-12-3167
- Gilsdorf A, Boxall N, Gasimov V, Agayev I, Mammadzade F, Ursu P,et al. Two clusters of human infection with influenza A/H5N1 virus in the Republic of Azerbaijan. Eur Surveill. 2006; 11: 122-126. DOI: 10.2807/esm.11.05.00620-en
- FAO: Despite many successes, avian influenza still threatens: FAO calls for sustained action on H5N1 and emerging infections. In FAO Media Centre, Rome. 2010. https://bit.ly/2D8JoU2
- Swayne DE. Avian influenza. In: Foreign animal diseases. Boca Raton, FL: United States Animal Health Association. 2008. pp. 137-146.
- OIE. Avian Influenza. Manual of Diagnostic Tests and Vaccines for Terrestrial Animal. Paris, France. 2005.
- Gohrbandt S, Veits J, Breithaupt A, Hundt J, Teifk J, Stech O, et al. H9 avian influenza reassortant with engineered polybasic cleavage site displays a highly pathogenic phenotype in chicken. J Gen Virol. 2011; 92: 1843-1853. DOI: 10.1099/vir.0.031591-0
- Swayne DE, Suarez DL. Highly pathogenic avian influenza. Rev Sci Tech. 2000; 19: 463-468.
- Rohm C, Horimoto T, Kawaoka Y, Suss J, Webster RG. Do hemagglutinin genes of highly pathogenic avian inßuenza viruses constitute unique phylogenetic lineages? Virology. 1995; 209: 664-670. DOI: 10.1006/viro.1995.1301
- Vahlenkamp TW, Teifke JP, Harder TC, Beer M, Mettenleiter, TC. Systemic Influenza virus H5N1 infection in cats after gastrointestinal exposure. Influenza Other Respir. Viruses. 2010; 4: 379-386. DOI: 10.1111/j.1750-2659.2010.00173.x
- Elbers A, Koch G, Bouma A. Performance of clinical signs in poultry for the detection of outbreaks during the avian inßuenza A (H7N7) epidemic in The Netherlands in 2003. Avian Pathol. 2005; 34: 181-187. DOI: 10.1080/03079450500096497
- Capua I, Mutinelli F. Low Pathogenicity (LPAI) and highly Pathogenic (HPAI) avian influenza in turkeys and chicken. In: Capua, I. and Mutinelli, F. eds. A Colour Atlas and Text on Avian Influenza, Papi Editore, Bologna. 2001; 13-20.
- Nakatani H, Nakamura K, Yamamoto Y, Yamada M, Yamamoto Y. Epidemiology, pathology, and immunohistochemistry of layer hens naturally affected with H5N1 highly pathogenic avian infuenza in Japan. Avian Dis. 2005; 49: 436-441. DOI: 10.1637/7304-110504R1.1
- Kwon YK, Joh SJ, Kim MC, Sung HW, Lee YJ, Choi JG, et al. Highly pathogenic avian influenza (H5N1) in the commercial domestic ducks of South Korea. Avian Pathol. 2005; 34: 367-370. DOI: 10.1080/03079450500181257
- Gordan RF. Poultry disease, fowl plague. 2nd ed. Bailliere Tindal, first Anne’s RP, Eastbaurne, East Sussex BN213UN. 1977; pp. 94-46
- https://bit.ly/3jRwH0P
- Spackman E, Pedersen JC, Mckinley ET, Gelb J. Optimal specimen collection and transport methods for the detection of avian Influenza virus and Newcastle disease virus. BMC Vet Res. 2013; 9: 35. DOI: 10.1186/1746-6148-9-35
- Swayne DE, Suarez DL, Sims LD. Influenza. In: Diseases of Poultry, 13th ed. In: Swayne DE, Glisson JR, McDougald LR, Nair V, Nolan LK, Suarez DL. Editors. Ames, Iowa, USA: Wiley-Blackwell. 2013. pp. 181-218.
- Chua T, Ellis T, Wong C, Guan Y, Ge S, Peng G, et al. Performance evaluation of five detection tests for avian influenza antigen with various avian samples. Avian Dis. 2007; 51: 96-105. DOI: 10.1637/0005-2086(2007)051[0096:PEOFDT]2.0.CO;2
- Suarez DL, Das A, Ellis E. Review of rapid molecular diagnostic tools for avian Influenza virus. Avian Dis. 2007; 51: 201-208. DOI: 10.1637/7732-101006-REGR.1
- Das A, Spackman E, Senne D, Pedersen J, Suarez D. Development of an internal positive control for rapid diagnosis of avian Influenza virus infections by real-time reverse transcription-PCR with lyophilized reagents. J Clin Microbiol. 2006; 44: 3065-3073. DOI: 10.1128/JCM.00639-06
- Espanol. Avian influenza: Current H5N1 situation. In avian influenza (bird flu). CDC. 2007; 1-4. https://bit.ly/30XylVQ
- Liu T, Bi Z, Wang X, Li Z, Ding S, Bi Z,et al. One family cluster of avian influenza A(H7N9) virus infection in Shandong, China. BMC Infect Dis. 2014; 14: 98. DOI: 10.1186/1471-2334-14-98
- Meng Z, Han R, Hu Y, Yuan Z, Jiang S, Zhang X, Xu J. Possible pandemic threat from new reassortment of influenza A (H7N9) virus in China. Euro Surveill. 2014; 19: 20699. DOI: 10.2807/1560-7917.es2014.19.6.20699
- Chen T, Zhang R. Symptoms seem to be mild in children infected with avian influenza A (H5N6) and other subtypes. J Infect. 2015; 71: 702-703. DOI: 10.1016/j.jinf.2015.09.004
- Pan M, Gao R Lv Q, Huang S, Zhou Z Yang L, et al. Human infection with a novel highly pathogenic avian influenza A (H5N6) virus: Virological and clinical findings. J Infect. 2016; 72: 52-59. DOI: 10.1016/j.jinf.2015.06.009
- Zhang W, Wan J, Qian K, Liu X, Xiao Z, Sun J, et al. Clinical characteristics of human infection with a novel avian-origin influenza A(H10N8) virus. Chin Med J. 2014; 127: 3238-3242. PubMed: https://pubmed.ncbi.nlm.nih.gov/25266520/
- Abdelwhab E, Veits J, MettenleiterC. Prevalence and control of H7 avian Influenza viruses in birds and humans. Epidemiol Infect. 2014; 142: 896-920. DOI: 10.1017/S0950268813003324
- Cong Y, Pu J, Liu Q, Wang S, Zhang G, Zhang X,et al. Antigenic and genetic characterization of H9N2 swine Influenza viruses in China. J Gen Virol. 2007; 88: 2035-2041. DOI: 10.1099/vir.0.82783-0
- Smith GJD, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic Nature. 2009; 459: 1122-1125. DOI: 10.1038/nature08182
- Vana, G, Westover K. Origin of the 1918 Spanish Influenza virus: a comparative genomic analysis. Mol Phylogenet Evol. 2008; 47: 1100-1110. DOI: 10.1016/j.ympev.2008.02.003
- Swayne DE. Understanding the ecology and epidemiology of avian infl uenza viruses: implications for zoonotic potential. In: Brown CC, Bolin CA. Editors. Emerging Diseases of Animals. Washington, D.C: ASM Press; 2000. pp. 101-130. https://bit.ly/2PaRQop
- Kilbourne ED. Influenza pandemics of the 20th Century. Emerg Infect. Dis. 2006; 12: 9-14. DOI: 10.3201/eid1201.051254
- Dunham EJ, Dugan VG, Kaser EK, Perkins SE, Brown IH, Holmes EC, et al. Different evolutionary trajectories of European avian-like and classical swine H1N1 influenza A viruses. J Virol. 2009; 83: 5485-5494. https://bit.ly/2WZcqwf
- Liu J, Bi Y, Qin K, Fu G, Yang J, Peng J, et al. Emergence of European avian influenza virus-like H1N1 swine influenza A viruses in China. J Clin Microbiol. 2009; 47: 2643-2646. DOI: 10.1128/JCM.00262-09
- Sinha N, Roy A, Das B, Das S, Basak S. Evolutionary complexities of swine flu H1N1 gene sequences. Biochem Biophys Res Commun. 2009; 390: 349-351. DOI: 10.1016/j.bbrc.2009.09.060
- Zhu H, Lam T, Smith D, Guan Y. Emergence and development of H7N9 Influenza viruses in China. Curr Opin Virol. 2016; 6: 106-113. DOI: 10.1016/j.coviro.2016.01.020
- Durrheim D, Ferson M. Preparing for the inevitable-an influenza. NSW. Public Health Bull. 2006; 17: 97-98. DOI: 10.1071/nb06023
- WHO. Evolution of H5N1 avian Influenza viruses in Asia. Emerg Infect Dis. 2005; 11: 1515-1521. DOI: 10.3201/eid1110.050644
- https://bit.ly/30eg33L
- WHO. Questions and answers on avian influenza in relation to animal, food and water. In Food Safety. 2007; 114.
- Gao Y, Zhang Y, Shinya K, Deng G, Jiang Y, Li Z, et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian Influenza viruses in a mammalian host. PLoS Pathog. 2009; 5: 1000709. https://bit.ly/3f7ZuKP
- WHO. Avian influenza (“bird flu”) fact sheet. 2014.
- Uyeki TM. Human infection with highly pathogenic avian influenza A (H5N1) virus: Review of clinical issues. Clin Infect Dis. 2009; 49: 279-290. DOI: 10.1086/600035
- Gao H, Lu H, Cao B, Du B, Shang H, Gan J, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013; 368: 2277-2285. DOI: 10.1056/nejmoa1305584
- Virlogeux V, Li M, Tsang T, Feng L, Fang V, Jiang H, et al. Estimating the distribution of the incubation periods of human avian influenza A(H7N9) virus infections. Am J Epidemiol. 2015; 182: 723-729. DOI: 10.1093/aje/kwv115
- Chan PK. Outbreak of avian influenza A (H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis. 2002; 34: 58-64. DOI: 10.1086/338820
- Tran TH, Nguyen TL, Nguyen TD, Loung TS, Pham PM, Nguyen VC. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004; 350: 1179-1188. DOI: 10.1056/NEJMoa040419
- Lasley FA. Economics of avian influenza: Control vs noncontrol. In: Proceedings of the Second International Symposium on Avian Influenza, Beard CW. U.S. Animal Health Association, Richmond, Virginia. 1986; pp. 390-399.
- EC: Impact assessment avian influenza (COM171). 2005.
- Lee CW, Suarez DL. Avian Influenza virus: Prospects for prevention and control by vaccination. Anim Health Res Rev. 2005; 6: 1-15. DOI: 10.1079/ahr2005101
- Oner A, Bay A, Arslan S, Akdeniz H, Sahin H, Cesur Y, et al. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N Engl J Med. 2006; 355: 2179-2185. DOI: 10.1056/NEJMoa060601
- Yalcin C. Market impact of HPAI outbreaks: A rapid appraisal process-turkey. In The Market and Trade Dimensions of Avian Influenza. Rome, FAO. 2006. pp. 1-28. https://bit.ly/3hNtOw9
- a. Otte J, Hinrichs J, Rushton J, Roland-Holst D, Zilberman D. Impacts of avian Influenza virus on animal production in developing countries. CAB Rev: Perspect Agric Vet Sci Nutr Nat Resour. 2008; 3: 1-18. https://bit.ly/2CVXQyP
- Dunning J, Baillie J, Cao B, Hayden F. Antiviral combinations for severe influenza. Lancet Infect Dis. 2014; 14: 1259-1270. DOI: 10.1016/S1473-3099(14)70821-7
- Marzoratti L, Iannella H, Gomez V, Figueroa SB. Recent advances in the diagnosis and treatment of influenza pneumonia. Curr Infect Dis Rep. 2012; 14: 275-283. DOI: 10.1007/s11908-012-0257-5
- Hayden FG, Hay AJ. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr. Top. Microbiol. Immunol. 1992; 176: 119-30. DOI: 10.1007/978-3-642-77011-1_8
- Bossart P, Carson M, Babu Y, Smith C, Laver W, Air G. Three dimensional structure of influenza A N9 neuraminidase and its complex with the inhibitor 2-deoxy 2,3 dehydro-N-acetyl neuraminic acid. J Mol Biol.1993; 232: 1069-1083. https://bit.ly/306XLBj
- Von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, et al. Rational design of potent sialidase-based inhibitors of Influenza virus replication. Nature. 1993; 363: 418-423. DOI: 10.1038/363418a0
- Colman PM, Hoyne PA, Lawrence, MC. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with Influenza virus neuraminidase. J Virol. 1993; 67: 2972-2980. DOI: 10.1128/JVI.67.6.2972-2980.1993
- OIE. Terrestrial animal health code Paris: OIE. Avian influenza. 2014. https://bit.ly/39EIjzx
- USGS (2005): National Wildlife Health Center. Wildlife Health Bulletin.
- Guan J, Chan M, Grenier C, Wilkie D, Brooks B, Spencer J. Survival of avian influenza and Newcastle disease viruses in compost and at ambient temperatures based on virus isolation and real-time reverse transcriptase PCR. Avian Dis. 2009; 53: 26-33. DOI: 10.1637/8381-062008-Reg.1
- Beard CW. Avian influenza. In: Foreign animal diseases.Richmond, VA: United States Animal Health Association. 1998; 71-80.
- Espanol. Avian flu CDC. 2015. https://bit.ly/3jNS2If
- Avian Influenza Vaccines: Focusing on H5N1 High Pathogenicity Avian Influenza (HPAI). CAST. 2007. https://bit.ly/39xjNAl
- Sherrilyn Wainwrighta , Carlene Trevenneca , Filip Claesa ,Moises Vargas-Terana , Vincent Martina , Juan Lubrotha. Highly pathogenic avian influenza in Mexico. FAO: Empress Watch. 2012. https://bit.ly/2X166EC
- Saad MD, Ahmed LC, Gamal-Eldein MA, Fouda MK, Khalil FM, Yingst SL, et al. Possible Avian Influenza (H5N1) from Migratory bird, Egypt. Emerg Infec Dis. 2007; 13: 1120-1121. DOI: 10.3201/eid1307.061222
- Van der Goot J, Koch G, de Jong MCM, van Boven M. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc Natl Acad Sci U S A. 2005; 102: 18141-18146. DOI: 10.1073/pnas.0505098102
- Wright PF, Webster RG. Orthomyxoviruses. In: Griffin DE. Editors. Fields Virology. Philadelphia, PA, USA: Lippincott Williams and Wilkins. 2001. pp. 1533-1579.
- Alemu D, Degefe T, Ferede S, Nzietcheung S, Roy D. Overview and background paper on Ethiopia’s poultry sector: Relevance for HPAI research in Ethiopia. Department for International Development (DFID) Pro-poor Highly Pathogenic Avian Influenza (HPAI) Risk Reduction Strategies Project Africa/Indonesia Region Report No. 1.Washington, D.C. International Food Policy Research Institute. 2008. https://bit.ly/3jJ8MQZ
- Bush J. The threat of avian flu predicted impacts on rural livelihoods in Southern Nation, Nationalities and Peoples Region (SNNPR), Ethiopia. The Food Economy Group (FEG) Consulting. 2006. https://bit.ly/2P2yOAA
- Berhane Y, Tefera A. Avian flu pandemic threat: Why is Ethiopia considered at risk? Ethiop J Health Develop. 2005; 19: 165-166.
- FAO: Poultry sector country review food and agriculture organization of the united nations. Animal production and health division. 2008. https://bit.ly/2BB0o4S
- Hayden F, Palese P. Influenza virus. In: Richman DD, Whitley RJ, Hayden FG. Clinical Virology. Washington, DC, USA: ASM Press. 2002; pp. 891-920.
- Markwell DD, Shortridge KF. Possible waterborne transmission and maintenance of infl uenza viruses in domestic ducks. Appl Environ Microbiol. 1982; 43: 110-115. DOI: 10.1128/AEM.43.1.110-115.1982
- McLeod A, Morgan N, Prakash A, Hinrichs J, FAO. Economic and social impact of avian influenza. Rome: FAO Emerg Cent Transbound Anim Dis Oper. 2005. https://bit.ly/3ga0Gil
- Nuradji H, Bingham J, Lowther S, Wibawa, H, Colling A, Long NT, et al. Comparative evaluation of feathers, oropharyngeal swabs, and cloacal swabs for the detection of H5N1 highly pathogenic avian influenza infection in experimentally infected chickens and ducks. J Vet Diagn Invest. 2015; 27: 704-715. DOI: 10.1177/1040638715611443
- WHO: Influenza. 2017. https://bit.ly/2Ew4sod
- WHO. Recommendations for influenza vaccines. World Health Organization: Geneva. 2007.
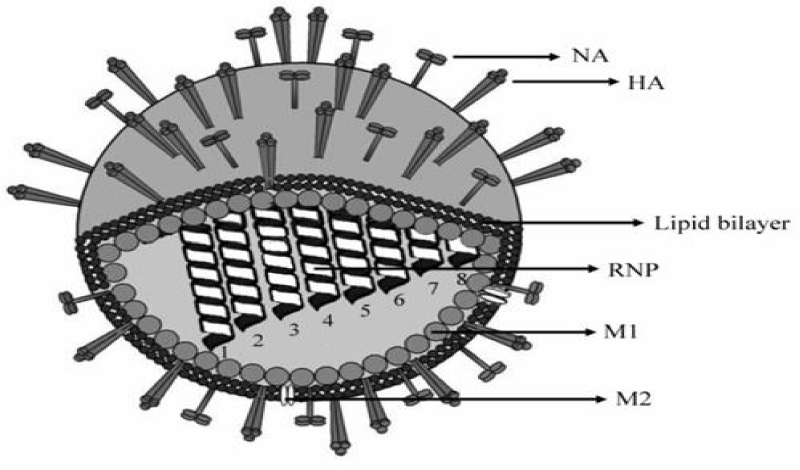
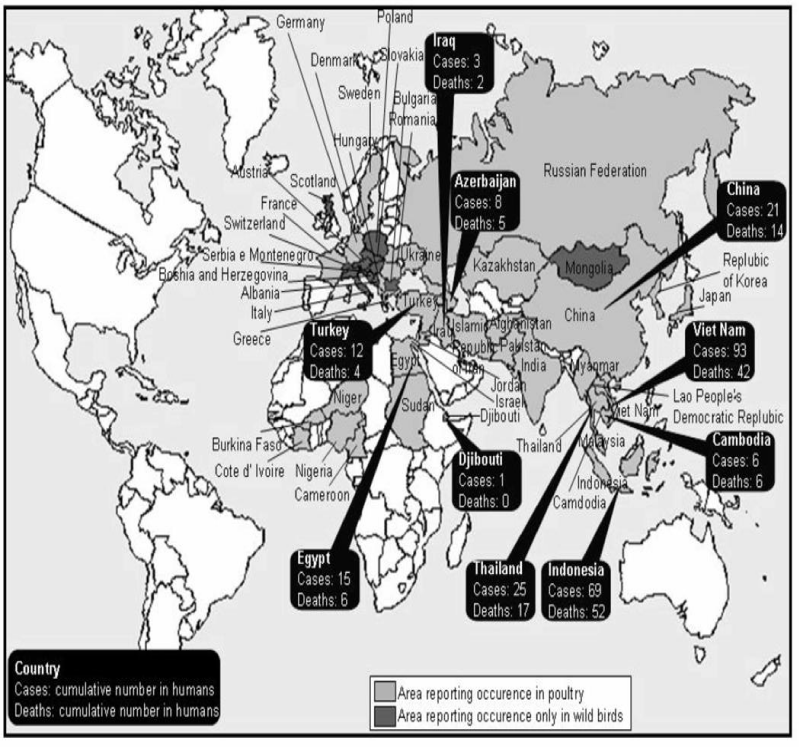
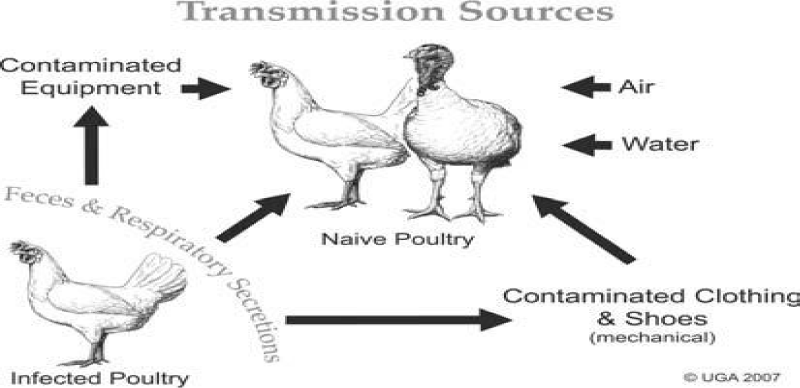
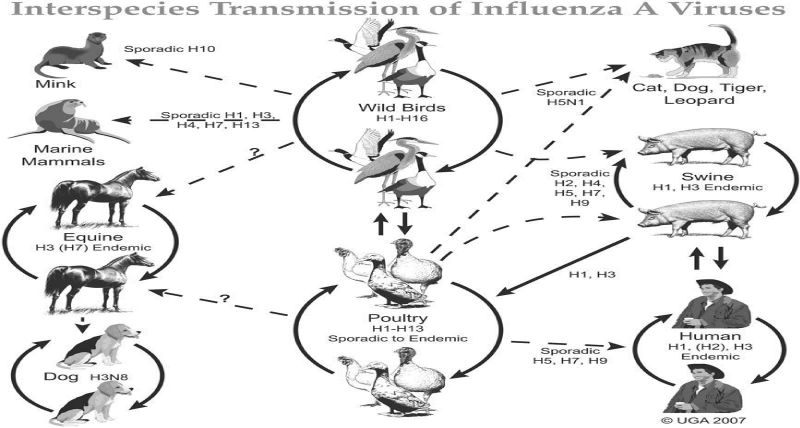
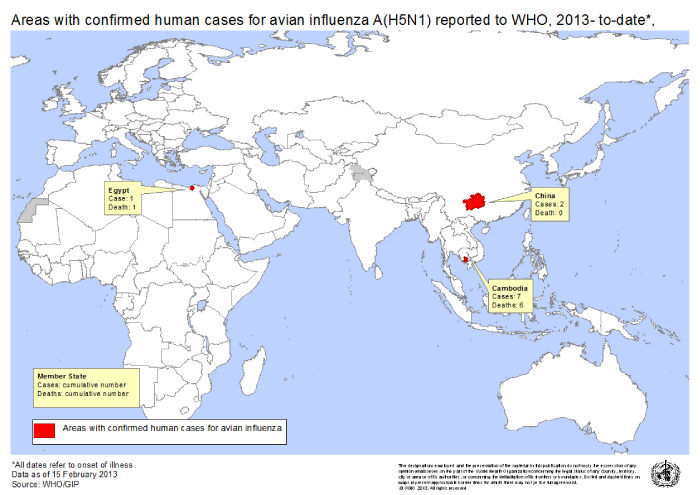

Sign up for Article Alerts