Cutaneous (wound) diphtheria by Nontoxigenic Corynebacterium diphtheriaevar. mitis - A Case Report?
SR Shah*, R Das and Lahiri KK
Department of Microbiology, BVDU Medical College, Pune. 411043 (India.)
*Address for Correspondence: SR Shah, Department of Microbiology, BVDU Medical College, Pune. 411043. (India.), Tel: +919-823-050-566; E-mail: sunilratilal@yahoo.co.in
Submitted: 20 November 2017; Approved: 16 December 2017; Published: 19 December 2017
Citation this article: Shah SR, Das R, Lahiri KK. Cutaneous (wound) diphtheria by Nontoxigenic Corynebacterium diphtheriaevar. mitis - A Case Report. Int J Virol Infect Dis. 2017;2(1): 025-029.
Copyright: © 2017 Shah SR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Cutaneous diphtheria; Nontoxigenic Corynebacterium diphtheriaevar. mitis ; Augmentin
Download FullteXt PDF
Corynebacterium diphtheria is considered now as a remerging pathogen. Several outbreaks of cutaneous diphtheria in developing countries are reported with rise in cases. Nondiphtherial corynebacteria are commonly involved in cutaneous diphtheria. However, cutaneous diphtheria caused nontoxigenic strains of Corynebacterium diphtheriae biovars have been seldom reported previously. We are addressing a rare case of cutaneous (wound) diphtheria in 36 years healthy male with a nonhealing wound, representing an area nonendemic for cutaneous diphtheria. Patient had a history of trauma and presented with a wound on supra malleolar region. Often coinfection exists in such wounds, and diagnosing a lesion as cutaneous diphtheria solely depends on demonstration and isolation of the Corynebacterium diphtheriae from it. Unusual isolation of nontoxigenic strain of Corynebacterium diphtheriaevar. mitis makes this case more interesting. Organisms were isolated and identified by routine cultural, automated and molecular methods. Patient responded well with oral Augmentin [Oxacillin (500mg.) plus potassium clavulanate (125mg.)] and Chlorhexidine acetate (0.5%) dressings. No post diphtheritic sequelae were observed. This case reports highlights the role of laboratory tests to isolate, differentiate and to detect the toxigenic nature of Corynebacterium species from a nonhealing ulcers, emphasizing early and accurate diagnosis can have a significant impact on clinical outcome in an individual with cutaneous diphtheria. Detection of such cases can also be a concern, for the community health.
Introduction
Nontoxigenic Corynebacterium diphtheriae are known to cause infections of skin, soft tissue, and sometimes invasive infections. Cutaneous diphtheria is a chronic, nonhealing ulcerative clinical entity, prevalent in endemic areas of tropics. Its incidence is on rise in developed countries, despite the mass immunization programmes [1]. It is a neglected clinical entity and seldom reported. Respiratory and cutaneous diphtheria cases are infectious and complementary to each other’s dissemination and may be associated with complications. We should be alert on detection of either of such a case in the view of the resurgence of respiratory diphtheria. Cutaneous diphtheria is found in patients coming from marginalized social groups with some prevailing underlying conditions. It is increasingly found in poor inner-city dwellers. Isolation & reporting of such organisms is important as non-toxigenic strains of Corynebacterium diphtheriae have the potential to lead to cardiac & renal complications. With same awareness, we are addressing a rare case of cutaneous (wound) diphtheria caused by non-toxigenic strains of Corynebacterium diphtheriaevar. mitis in a healthy male with nonhealing ulcer, representing a non-endemic area. Lab diagnosis plays an important role in detection of Corynebacterium diphtheriae in a non-healing ulcer. This case report highlights the importance of various microbiological methods used for early identification, speciation and determining toxigenicity that might be imperative to manage the case considering the lethal sequelae of cutaneous diphtheria. Unusual isolation of nontoxigenic strain of Corynebacterium diphtheriaevar. mitis in this case has taken us by surprise.
The case
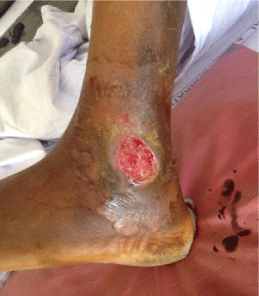
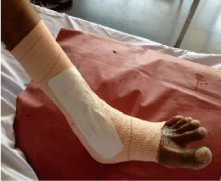
36 yrs old healthy male from Katraj-Pune, was admitted to tertiary care centre at Pune (India). He complained of nonhealing wound, persistent for last one month. Patient gave a history of fall from bike with a small wound which had increased to existing size over the period. Patient also complained of oedema around the wound, with reduced sensation over the wound and pain in leg for last 5 days. There was no history of fever, chills or any previous illness. Patient was not known to be hypertensive, diabetic or alcoholic with no history of any dermatological ailments. History of past immunization was obscure. He worked in a steel fabrication workshop. On examination the wound was located on lateral side of left leg over malleolus region [Figure 1]. There was a single ulcerated wound, (5 X 3 cms.) oval with demarcated undermined edges. It had a reddish base with serosanguinous and tenacious slough. Wound showed few yellow to greyish white pseudomembrane resembling pockets. There was no eschar. The wound was insensitive to touch. X-ray - PA/Oblique--showed soft tissue lacerations on lateral aspect of malleolus. All the parameters were in normal limits.
Methods
Microbiological methods
Day 1: Two pus swabs were collected for Gram’s / ZN and culture sensitivity. No operative procedure was planned. There was no case referral. Daily dressing with sterile saline was advised. No antibiotics were administered till lab report was received. Swabs received in lab were having blood tinged purulent material.
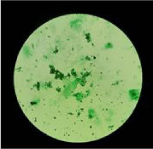
Microscopy results: ZN staining was negative for AFB. Gram stain revealed few pus cells and gram positive slender rods of varying size, having V and L shapes arrangements. Few bacilli showed faint intracytoplasmic granules. No other organisms were seen. On suspicion Albert’s stain was carried out which showed similar picture. [Figure 2]. Provisional report of ‘Gram positive bacilli suggestive of corynebacterial spp. (Diphtheroids)’ was sent. Further entire processing of sample was carried out in biosafety class II cabinet with all precautions [1]. To exclude the possibility of contamination second sample was also processed simultaneously. Swabs were Cultured on Sheep Blood agar /MacConkey’s agar Loeffler’s serum slopes and incubation aerobically at 37 0C.
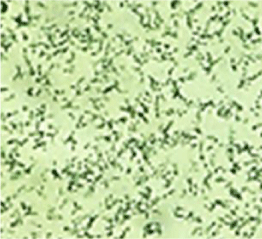
Day 2: Blood agar showed 1-2 mm small, greyish white, shiny, pure colonies with faint haemolysis, which was more evident after 48 hrs of incubation (Figure 3). Haemolytic nature is seen in Corynebacterium diphtheriae biotype - mitis and Corynebacterium haemolyticum, Corynebacterium afermentans. MacConkey’s agar- showed no growth. Loeffler’s serum showed white creamy colonies whose culture smear showed peculiar appearance of corynebacteria (Figure 4). Second sample yielded similar results. Susceptibility test by Disk Diffusion was done by inoculating a 0.5 McFarland suspension of the organism onto 5% sheep blood agar and incubated for 24h in ambient air (Interpretation criteria for Staphylococcus spp), recommended by the NCCL Standards were followed [2].
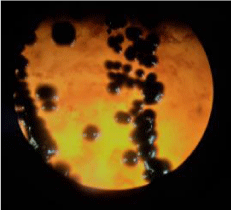
Day 3: Potassium tellurite medium grew, black coloured poached egg colonies [Figure 5]. Tinsdale’s agar grew black coloured colonies [Figure 6]. Culture was subjected for species Identification by automated technique (Phoenix BD 100). Urease test was performed to rule out Corynebacterium ulcerans and Corynebacterium pseudotuberculosis and Diphtheroids. REVERSE - CAMP test was negative, suggesting Corynebacterium diphtheriae excluding Corynebacterium ulcerans and Corynebacterium pseudotuberculosis [Figure 7]. Antibiotic Susceptibility Testing Results: Showed Penicillin–S / Teicoplanin-S / Amoxiclav-S / Trimethoprim-sulfamethoxazole-S / Erythromycin-R / Gentamicin-S / Clindamycin – R / Rifampicin- S / Ciprofloxacin-S and Vancomycin-S ---- [S = Susceptible, R=Resistant]
Day 4: Elek’s test carried out for toxogenicity was negative, suggesting nontoxogenic nature of the isolate. Sequencing and detection of a segment of toxgene by PCR (Polymerase chain Reaction) was carried out at NCCS (National Centre for Cell sciences, Savitribai Phule University Campus, Pune India) and was negative, that confirmed the nontoxigenic nature of the strain. Isolate was also identified by MALDI TOF (Matrix Assisted Laser Desorption Ionisation-Time of Flight mass spectrometry) technique to support earlier findings.
Results
Final identification of the isolate was established as Corynebacterium diphtheriaevar. mitis . Clinical diagnosis was established as cutaneous (wound) diphtheria with nonhealing ulcer. No other differential diagnosis was considered.
Treatment
Patient was isolated in ward from day 1 of admission and as history of immunization was obscure a booster dose of DTP vaccine was given. The wound was cleaned with sterile saline daily and dressed in chlorhexidine acetate gauze (Bactigrass) before discharge [Figure 8]. Patient was treated with oral Augmentin [Amoxicillin (500mg). + Clavulanic acid (125 mg)]-1BD x 5 days from second day itself after we took second sample and gave a provisional report. Clinician was consulted who continued same treatment after receipt of ABST report…3rd day supplemented with Tab PAN 40mg. 1ODx 5 days and Tab Chymoral forte 1 TDS X 5 days. The case was followed for 5 days and later once every week. The wound completely healed after 2 ½ month. No post-diphtheric sequelae was observed in this case.
Discussion
Cutaneous diphtheria prevails over respiratory in tropic and subtropical climates. Cutaneous diphtheria is endemic in African, South Pacific Mediterranean, and Asian regions [3]. UK, Russia, Trinidad, Northern Australia have reported breakouts of cutaneous diphtheria in recent yrs [4]. There is less data available on cutaneous diphtheria alone in India, even though any type of diphtherial infection is notifiable. There is a need for epidemiological survey of the area if the patient represents a non-endemic area, as seen in this case. Possible Coryne forms causing skin ulcers include all biovars of Corynebacterium diphtheriae (Toxigenic and Non-toxigenic), Corynebacterium ulcerans, Corynebacterium tenuis, Corynebacterium pseudotuberculosis , Corynebacterium minutissimum, Corynebacterium jekeium. The causative agent in present case was nontoxigenic Corynebacterium diphtheriaevar. mitis . Humans are the only reservoir of Corynebacterium diphtheriae [5]. Respiratory diphtheria and carriers can be a source for cutaneous lesions (ratio being 95 carriers for 5 clinical cases) [6]. Transmission of cutaneous diphtheria can occur by contact with respiratory secretions, infected skin lesions. Cutaneous diphtheria is more infectious than respiratory and the ulcerated sites have been shown to contaminate the environment including hospitals [7]. Wound diphtheria, is a secondary type of cutaneous diphtheria occurring after a skin trauma (abrasions, lacerations, burns) involving exposed areas of the feet, legs and hands [8] Patient in this case report had a history of fall from bike, but how the wound was infected remains unclear. Whether the isolate obtained in this case had an endogenous or exogenous origin remains unclear. Cutaneous diphtheria is ignored as it is not routinely screened for and is often under diagnosed. Gram’s stain results of wound swab with Gram positive diphtheria like organisms must not be interpreted as skin contaminants and abandoned. Our results of Gram staining were immediately informed to clinician as a part of alert reporting. Microbiological methods were employed in this case which identified the isolate as Corynebacterium diphtheriaevar. mitis . excluding other corynebacteria. The use of MALDI-TOF and Phoenix BD 100 results, confirmed the identity. The challenge was to find out the toxigenic nature of this isolate. PCR for detection of tox gene remains a rapid option but presence of tox gene doesn’t necessarily establish the toxigenicity of strain as it may not be expressed. Elek’s test is an easy and a better toxogenicity test. Elek’s test was negative for this isolate revealing its nontoxigenic nature. However non-toxigenic strains also can have fatal sequela and can’t be ignored as emphasized in this report. Non-toxigenic strains are on rise in UK and Europe and are known to cause local disease. Alexandra, Anna, Zasada et al, reported an increase in non- toxigenic Corynebacterium diphtheriae infections in Poland [9]. Recently cutaneous diphtheria due to Corynebacterium diphtheriae have been imported via migrant to UK, Sweden, Germany, Australia and New Zealand. Reynolds G.E. et al reported cutaneous diphtheria in two Afghanistan refugees from Auckland refugee settlement [10]. Kolios A.G. A et al reported 2 Eritrean patients with a similar clinical presentation of a cutaneous infection with nontoxigenic Corynebacterium diphtheriae and Corynebacterium striatum [11]. diphtheria toxins is the main virulent factor in Corynebacterium diphtheriae, however non-toxigenic isolates from invasive infections and cutaneous ulcers suggest that there is a possibility of another additional virulence factor. Toxin-mediated diphtheria has diminished due to vaccine but might have caused selective pressure, which has forced Corynebacterium diphtheriae to express or develop means of causing disease and virulence factors, other than toxin-linked molecular mechanisms. Melnikov V G et al, in their study in different parts of Russia observed that 13.8% nontoxigenic tox carrying strains belonged to biovar mitis [12]. In recent outbreak of cutaneous diphtheria in migrants in UK, 15 isolates out of 17 were nontoxigenic strains of mitis [13]. Nontoxigenic invasive strains have emerged in Australia, Canada, Germany, France and Vancouver [14]. This case also carries a nontoxigenic mitis variant of Corynebacterium diphtheriae. In highly immunized populations, toxigenic strains have virtually disappeared, although non-toxigenic strains may continue to circulate [15]. Studies show that nontoxigenic strains can acquire prophages from toxigenic strains [16]. For all above reasons the nontoxigenic isolate remains a potential danger and could be a silent killer. So was relevant in this case too. The patient was monitored by regular follow up to check for any post diphtheric sequelae. The antimicrobial susceptibilities of Corynebacterium species are usually unpredictable [17]. Isolate from this patient showed resistance to erythromycin, clindamycin and cotrimoxazole but was susceptible to penicillin, amoxiclav, gentamicin, vancomycin, and ciprofloxacin (by disk diffusion). Patient responded well with Augmentin [Amoxicillin-(500mg.) plus potassium clavulanate (125 mg.)] orally and chlorhexidine acetate (0.5%) dressings locally. No post diphtheric sequelae were observed. Cutaneous diphtheria of any nature can be a health problem for an individual, a community or Hospital environment. This case study highlights the fact that the Coryne forms are no longer just opportunistic pathogens but they are also becoming important pathogens.
- Biosafety in microbiological and biomedical laboratories laboratory, Section IV-Laboratory biosafety criteria. Centers for Disease Control and Prevention National Institutes of Health. 2016; 30-37. https://goo.gl/sh9iCN
- Wayne PA. National committee for clinical laboratory standards. Performance Standards for Antimicrobial Susceptibility Testing; 13th Informational Supplement. NCCLS. Document. 2003; 100-113.
- Leva Kantsone. Surveillance and outbreak report. Euro surveillance. 2016; 21.
- de Benoist AC, White JM, Efstratiou A, Kelly C, Mann G, Nazareth B, et al. Imported cutaneous diphtheria, United Kingdom. Emerg Infect Dis. 2004; 10: 511–515. https://goo.gl/8xhjAL
- Centre for Disease Control. Traveler’s Health: Yellow Book; Chapter 4: Prevention of specific infectious disease. https://goo.gl/dAXuiP
- Koopmans J S, Campbell J. The role of cutaneous diphtheria infections in a diphtheria epidemic. Infect Dis. 1975; 131: 239-244. https://goo.gl/Se5n1B
- A Berih. Cutaneous Corynebacterium diphtheriae-A traveller’s disease?. Can J Infect Dis. 1995; 6: 150-152. https://goo.gl/jsVyJM
- Vetrichevvel TP, Pise GA, Agrawal KK, Thappa DM. Cutaneous diphtheria masquerading as a sexually transmitted disease. Indian J Dermatol Venereol Leprol. 2008; 74: 187. https://goo.gl/sgskS6
- Aleksandra AZ, Milena BR, Sebastian W. Molecular epidemiology and antimicrobial susceptibility of strains isolated from past outbreaks and those currently circulating in Poland. International Journal of Infectious Diseases. 2010; 14: 907-912. https://goo.gl/oEiWGr
- Reynolds GE, Saunders H, Matson A, O'Kane F, Roberts SA, Singh SK, et al. Public health action following an outbreak of toxigenic cutaneous diphtheria in an Auckland refugee resettlement centre. Commun Dis Intell Q Rep. 2016; 40: 475-481.https://goo.gl/LcwA4s
- Kolios AGA, Cozzio A, Zinkernagel AS, French LE, Kündig TM. Cutaneous Corynebacterium infection presenting with disseminated skin nodules and ulceration. Case Rep Dermatol. 2017; 9: 8-12. https://goo.gl/ZZrcsb
- Mel'nikov VG, Kombarova SIu, Borisova OIu, Volozhantsev NV, Verevkin VV, Volkovoĭ KI, et al. Corynebacterium diphtheriae nontoxigenic strain carrying the gene of diphtheria toxin. Zh Mikrobiol Epidemiol Immunobiol. 2004; 1: 3-7. https://goo.gl/amDrSL
- Bray JP, Burt EG, Potter EV, Poon-King T, David PE. Epidemic diphtheria and skin infections in Trinidad. J Infect Dis. 1972; 126: 34-40. https://goo.gl/fAmQJL
- Romney MG, Roscoe DL, Bernard K, Lai S, Efstratiou A, Clarke AM. Emergence of an invasive clone of nontoxigenic Corynebacterium diphtheriae in the urban poor population of vancouver. Canada. J Clin Microbiol. 2006; 44: 1625-1629. https://goo.gl/jy7885
- Wilson AP. The return of Corynebacterium diphtheriae: The rise of non-toxigenic strains. J Hosp Infect. 1995; 30: 306-312. https://goo.gl/dqNza3
- Freeman VJ. Studies on the virulence of bacteriophage infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951; 61: 675-688. https://goo.gl/WmL5jG Vladimir Vicente Cantarelli, Brodt TC, Secchi C, Inamine E, Pereira Fde S. Cutaneous infection caused by Corynebacterium pseudodiphtheriticum. A microbiological report. Rev Inst Med Trop Sao Paulo. 2008; 50: 51-52. https://goo.gl/B5QUAK




Sign up for Article Alerts