Presence of Trichobilharzia Cercariae at Public Bathing Places of Natural Lakes in Schleswig-Holstein, Northern Germany?
Frauke Elbnik1*, Hermann Feldmeier2 and Regina Folster-Holst3
1Christian-Albrechts University, Kiel
2Charite University Medicine Berlin, Campus Benjamin Franklin, Institute of Microbiology and Infection Immunology, 12203 Berlin, Germany
3University Hospital Schleswig-Holstein Campus Kiel, Department of Dermatology, Venerology and Allergology, 24109 Kiel, Germany
*Address for Correspondence: Frauke Elbnik, Christian-Albrechts University, Kiel, Germany, Tel: +015-152-892-009; E-mail: fraukebutenschoen@web.de
Submitted: 02 December 2019; Approved: 10 December 2019; Published: 19 December 2019
Citation this article: Elbnik F, Feldmeier H, Folster-Holst R. Presence of Trichobilharzia Cercariae at Public Bathing Places of Natural Lakes in Schleswig-Holstein, Northern Germany. Int J Virol Infect Dis. 2019;4(1): 011-016.
Copyright: © 2019 Elbnik F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Cercariae; Schleswig-Holstein; water snails
Download Fulltext PDF
Background and Objective: Cercarial dermatitis is a parasitic disease occurring in temperate zones. In Central Europe it is caused by cercariae of the genus Trichobilharzia. These cercariae parasitize waterfowl, but sometimes penetrate into human skin and cause an allergic reaction. Reliable data on the occurrence of cercarial dermatitis does not exist. So the primary objective of this study was to determine the occurrence of cercariae that can cause cercarial dermatitis in humans in the public bathing places of Schleswig-Holstein.
Methods: Cercariae are released by infected water snails. To determine the occurrence of cercariae-emitting snails in Schleswig-Holstein, 155 public bathing places were visited and searched for fresh water snails. Family and genus of the collected snails were determined and the snails were examined for the shedding of cercariae, using a standard method and a newly developed method.
Results: In total, eight different families/genera of water snails were found. Overall at 55% of the bathing places cercariae-emitting snails were identified. The new detection method yielded more positive results compared to the standard method. Besides, the number of cercariae recovered by the new method was higher.
Conclusion: Since cercarial dermatitis is no notifiable disease, data about the prevalence is sparse. Overall cercariae occur in a great number of fresh water lakes in Schleswig-Holstein. They can be detected by standard method or in a shorter amount of time by the newly developed method. Nevertheless further studies will be necessary to evaluate possible advantages and disadvantages of the new method.
Background and Importance
Cercarial dermatitis (also called swimmer’s itch) is a neglected parasitic disease with distribution all over the world’s temperate zones. It is caused by cercariae of flukes of the family Schistosomatidae that normally parasitize waterfowl, such as ducks and geese [1]. The cercariae are released by infected freshwater snails, the intermediate host, and then attempt to penetrate into a final host. After successful penetration of the skin of a suitable host, cercariae transform into schistosomulae and later into adult flukes, eventually leading to waterfowl schistosomiasis, a systemic disease [2,3]. Occasionally, cercariae of the genus Trichobilharzia mistake the skin of humans or pets for the skin of waterfowl and then penetrate into the skin of a wrong host. However, in the skin of a wrong host the cercariae are in a biological impasse and get trapped, hence they cannot transform into schistosomula. This results in a local inflammation named cercarial dermatitis which starts with a tingling or burning sensation and then develops into itching maculae and papules [4-6].
Typically, cercarial dermatitis resolves within a couple of weeks [4,5]. Very rarely cercarial dermatitis develops into an abscess or circumscribed necrosis, presumably due to bacterial superinfection as a consequence of scratching the itchy lesion [7-9].
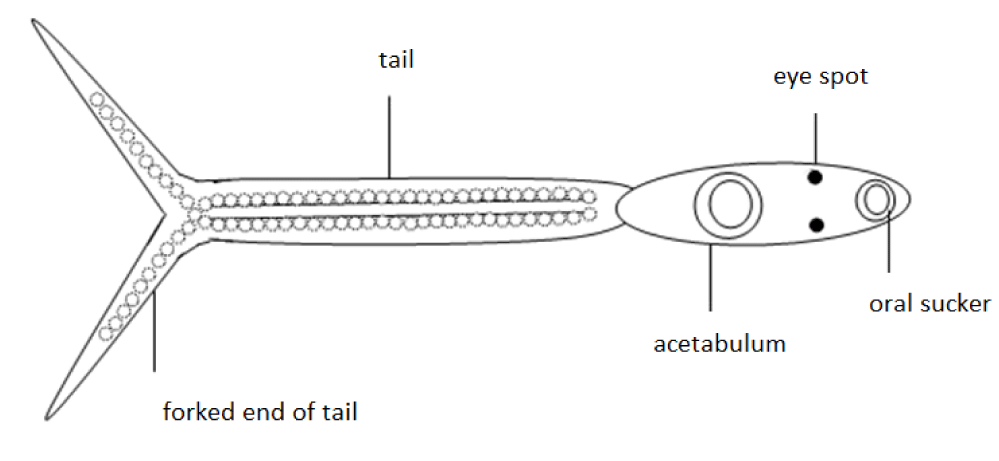
In Northern Germany cercarial dermatitis is commonly caused by cercariae of the genus Trichobilharzia, such as T. szidati, T. regenti and T. franki [10]. The cercariae appear in warm summer months in stagnant freshwater in great numbers. They show a typical microscopic appearance with a forked tail and two eye spots (Figure 1). Correct and specific determination of the species is only possible by molecular analysis [11-14].
Since cercarial dermatitis is not a notifiable disease there is no systematic recording of cases. Therefore appropriate information is sparse and mainly about certain regions or single water bodies. In such studies, a high prevalence of cercariae up to 44.9% has been found [15]. For Schleswig-Holstein there is no study of this kind this far.
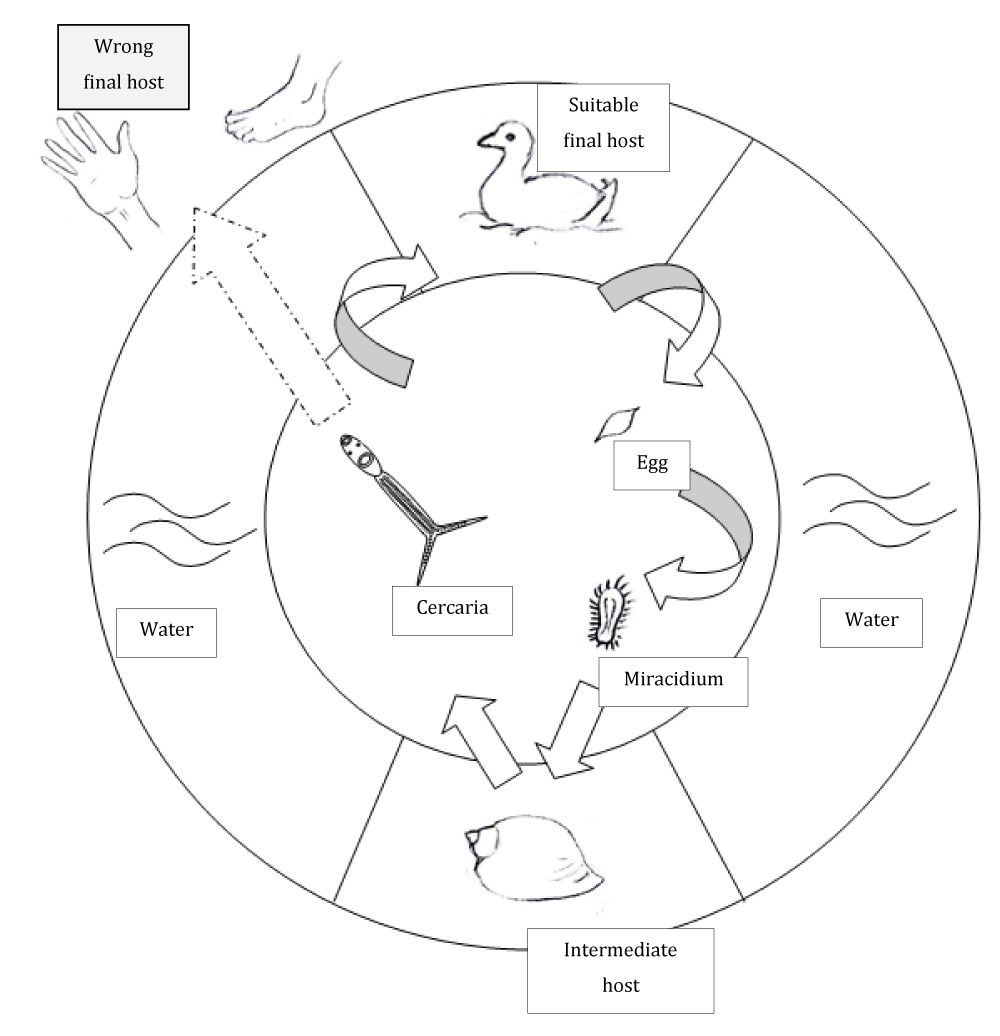
In a warm summer day, a single water snail can release up to 100.000 cercariae [16]. These swim around searching for a compatible host. Hosts are found by sensing of light and shade, temperature gradient and chemotaxis [17]. Fatty components of skin, especially ceramides seem to attract cercariae [17]. When cercariae have identified a suitable skin, they start the penetration process by emptying special secretion glands to make the corneal layer of the skin permeable [17-20]. Once inside the skin the cercariae penetrate into a small blood vessel. After reaching the bloodstream cercariae of T. szidati and T. franki travel to the digestive organs and the liver of waterfowl, whereas cercariae of T. regenti travel to the central nervous system [21,22]. Here, the larvae develop to adult worms and begin the production of eggs that are dispersed into water. Once in the water, miracidiae hatch from the eggs and search for a suitable freshwater snail [3,20,23]. The life circle is shown in figure 2.
In Central and Northern Europe cercarial dermatitis occurs from May to August when peak shedding of cercariae and the bathing season coincide [13,14,24]. Previous studies indicate that the density of Trichobilharzia cercariae is particularly high in shallow water during warm summer days indicating that the release of cercariae from intermediate hosts depends on water temperature [25]. As the snails’ species acting as intermediate hosts concentrate at the margin of natural water bodies where the water is shallow, public bathing places and the habitat of intermediate hosts overlap, posing bathers at risk to get infected [26,27].
The objective of the study was to determine the prevalence of the cercarial dermatitis in correlation to the Trichobilharzia cercariae at the fresh water bathing places of Schleswig-Holstein in 2014. To do so, we have sent questionnaires to the doctors of Schleswig-Holstein, including dermatologist, pediatricians and general practitioners. Furthermore all public bathing places were surveyed for snails and collected snails were examined for shedding cercariae using a standard procedure as well as a newly developed method.
Materials and Methods
To get information on reported cases of cercarial dermatitis in the past years, all public health district offices of Schleswig-Holstein were contacted. Additionally questionnaires were sent via the medical association to dermatologists, pediatricians and general practitioners. Also health insurances were contacted for data.
To get an overview of the prevalence of cercariae anyway, every bathing place in Schleswig-Holstein was surveyed for cercariae in 2014. There are 104 lakes with a total number of 155 public bathing places, distributed over eleven rural districts. The exact location of the bathing places were provided by the Ministry of Health and Social Services in Kiel. Data on weather, wind, presence of plants and soil were registered at every bathing place. Bathing places were visited between 8 a.m. and 6 p.m. and examined systematically. In Schleswig-Holstein public bathing zones are marked by floating chains of buoys. Snails were collected within these limits. Starting at one side, the entire bathing place was searched in a meandering pattern until the other side was reached.
When the water was clear, snails were collected by hand from plants, floating leaves, stones and wood, or were caught off the ground with a scoop. If visibility was limited by algae or sediment, plants were striped from the ground to the surface by hand and the scoop was used blindly. Collected soil was rinsed with clear water and searched for snails.
To allow comparison between bathing places, search time was limited deliberately. After the first snail was found the search was continued for exactly 15 minutes and every snail found was collected. If no snail was found within 30 minutes, the search was stopped and classified as not successful.
All snails were placed in plastic bags containing water from the corresponding bathing place and a few water plants. The bags were labeled with date, hour, identification number of bathing place and lake. A sufficient air circulation was ensured by leaving the bag open and putting them into stable basins to keep the content from spilling.
The bags were kept in shade and at environmental temperature. All snails were examined within eight hours after collection, but always on the day of collection. Snails which were kept for several days were fed with lettuce and customary forage for freshwater fish. Then they were released at the bathing place they had been collected.
The genus and species of every snail were determined using identification schemes provided by Jekel and Zick [28], Sturm [29] and Zettler [30].
To identify shedding of cercariae each snail was put into a glass bowl with a diameter of 7 cm filled with 15 ml of 25°C warm tap water [31]. The bowls were placed in sunlight for 15 minutes to provoke the shedding of cercariae (method modified after [11]). Then one milliliter of water was taken and put on a glass slide and examined through a microscope at tenfold magnification.
Cercariae were classified by the existence of eye spots and the shape of the tail according to Neuhaus [20], Jekel and Zick [28] and Faltynkova [8,32] (Figure 1). Only water samples containing cercariae with two eye spots and a forked tail were considered to contain Trichobilharzia cercariae.
Since the conventional method of detecting cercariae is time consuming and obviously not very sensitive, a new method was developed. As soon as snails begin to move around at the bottom of a glass bowl they produce a trail of slime to glide on. The slime was removed with a small wooden stick and transferred with a pipette to a slide and examined under tenfold magnification for the presence of cercariae.
Results
Since cercarial dermatitis is not a notifiable disease, the documented cases of cercarial dermatitis retrieved from the public health district offices of Schleswig-Holstein were fragmentary and not conclusive. The questionnaires designed for this study were sent to all resident dermatologists, resident pediatricians and general practitioners. Although they were sent via the medical association of Schleswig-Holstein, only two questionnaires were returned. The health insurances did also not provide any utilizable information.
All 155 official bathing places that were registered by the Ministry of Health and Social Services in Kiel were sampled. Snails could be found at 148 bathing places. In total 2311 snails were collected. Trichobilharzia cercariae were recovered from 341 snails (14.8%) from 85 bathing places (55%).
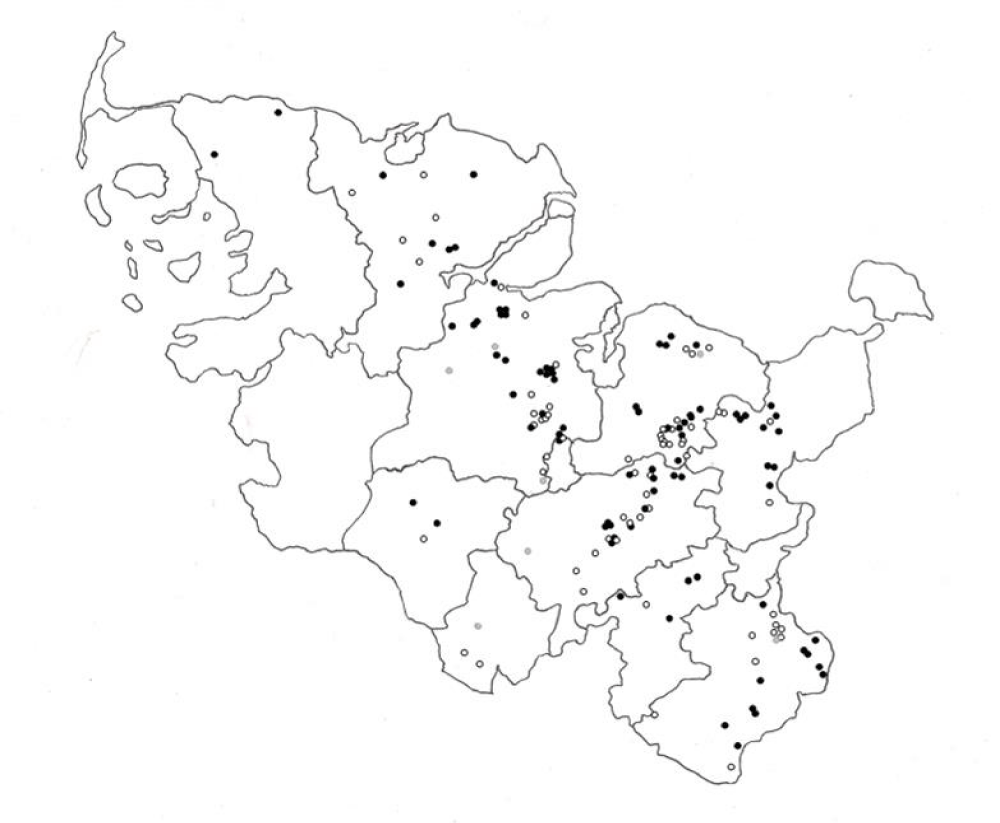
The spatial distribution of lakes with Trichobilharzia cercariae shedding snails is shown in figure 3. The collected snails belonged to the family of Lymnaeidae (genera Lymnaea sp. and Radix sp.), the family of Planorbidae (genera Planorbarius sp. and Planorbis sp.), the families of Bithynidae, Viviparidae and Physidae as well as the family of Nerthidae (Theodoxus sp.). Table 1 shows the different families and genera and their shedding of Trichobilharzia cercariae.
Most common were snails of the genus Lymnaea sp. (931out of 2211 snails, 40.3%). 249 of the Lymnaea sp shed cercariae (26.7%). So this genus of snails was the most common host for cercariae in this survey. 682 (73.2%) of Lymnaea sp. did not shed cercariae.
487 specimens of snails of the genus Radix sp. were found which equals 21.1% of all collected water snails. 69 (14.2%) of the tested Radix sp. shed cercariae what makes these snails the second leading genus. 418 (85.5%) of the collected Radix sp. did not shed cercariae. In total 10.7% of the found water snails were Radix sp.
Snails of the genus Planorbarius sp. were occasionally found. In total 127 specimens were collected. They contributed 5.5% of all water snails. 18 (14.2%) of the Planorbarius sp. shed cercariae. In 109 samples (85.8%) no cercariae could be found.
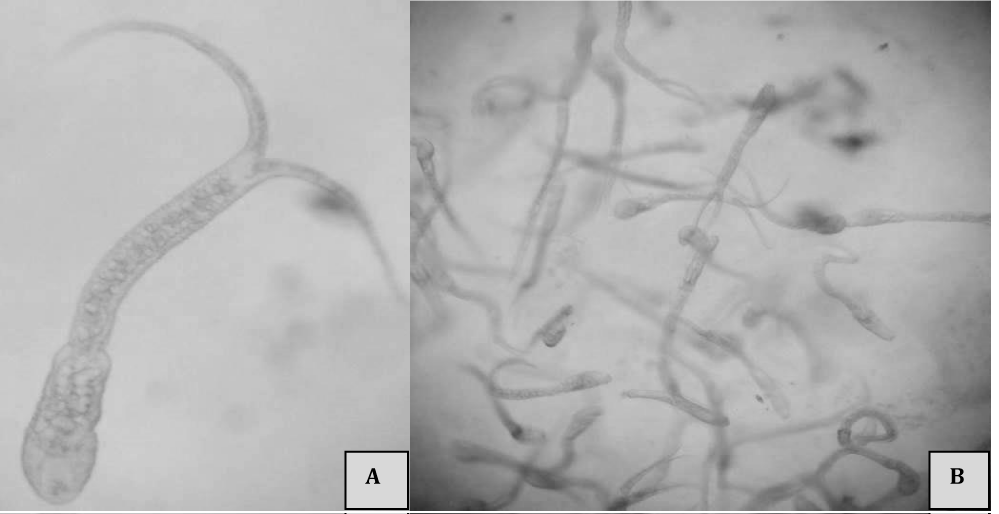
Table 1 also shows further snail species found and their shedding of cercariae. To verify whether the standard procedure yielded false negative results, for a couple of bathing places, shedding of cercariae was determined by the standard procedure and a method based on the examination of slime. Cercariae sticking in the slime could be easily identified (Figure 4). Cercariae tried to free themselves from the slime by abrupt movements of their tails. Despite of intense movement in some cases cercariae needed more than 20 minutes to escape from the slime. Table 2 compares both methods for the snails collected at the bathing place at the Lanker See.
Discussion
Trichobilharzia cercariae are the cause for cercarial dermatitis all over the world. The purpose of this research was to assess the prevalence of cercarial dermatitis in Schleswig-Holstein and correlate the prevalence with the distribution of cercariae in freshwater lakes used as bathing places in Schleswig-Holstein.
To evaluate the prevalence of cercarial dermatitis in Schleswig-Holstein, we retrieved existing data about cercarial dermatitis from every public health district office in Schleswig-Holstein. Furthermore we sent a questionnaire via the medical association to dermatologists, pediatricians and general practitioners. The data of the health district offices was fragmentary and therefore not conclusive. Only two questionnaires were returned, so this data also was not utilizable. Also there was no data provided by health insurances.
All 155 public bathing places were visited in 2014 and searched for fresh water snails in the peak bathing season. Different families/genera of water snails were found to shed cercariae. Snails of the family of Lymnaeidae most frequently shed cercariae, counting up to a total of 1418 specimen, 62.5% of all found snails. Snails of this family are known to be hosts for many different types of cercariae [8,15]. In previous surveys Lymnaea sp were found to be infected in 14.1% to 26.3% [8,20,33]. At single locations up to 44.9% of these snails shed cercariae. Hence an infection rate of 26.8% found in this study seems plausible [15]. The genera of Radix sp. and Planorbarius sp. were infected in 14.2% of the cases. Water snails of the families Physidae and Bithyniidae were not found to be infected. This contrasts with a previous report [33].
The examination of slime identified substantially more cercariae-shedding snails. Hence, the number of bathing places in Schleswig-Holstein at risk for acquiring cercarial dermatitis should be considered higher than the 85 bathing places identified with the standard method.
The presence of water snails and cercariae is determined by several factors. The appearance of cercariae requires sunlight and a high water temperature. Snails rely on plants and forage for their survival. These environmental conditions are different from year to year and also within a year. Therefore, a negative result for identifying cercariae at the time of study does not guarantee that a bathing place will be free of cercariae in the future [11,26]. Hence, our results should be seen as a snapshot taken at the peak season of cercarial dermatitis in 2014.
Data Availability
The GPS coordinates and identification codes of the bathing places were provided by the “Amt für Soziale Dienste” Kiel and are included in table 1. Further geographical data used in Figure 3 were supplied by the Institute of Geology, University of Kiel, under license. Requests for access to these data should be made to www.LVermGeoAH.schleswig-holstein.de
Declaration
No previously published material was used for this article. Contents of the manuscript have not been previously published and are not currently submitted elsewhere. I accept responsibility for the scientific integrity of this work. All previously listed authors have seen and approved of the manuscript and will sign off on any subsequent manuscript revisions.
Acknowledgement
We thank the department “Amt fur Soziale Dienste” Kiel and the Institute of Geology, University of Kiel for providing valuable geographical data and coordinates. Furthermore we thank the Department of Dermatology, Venerology and Allergology of the Christian-Albrechts University, Kiel for enabling us to do this field research.
Special thanks go to our friends and families for their constant support.
- Cort WW. Schistosome dermatitis in the United States (Michigan). JAMA. 1928; 90: 1027-1029. http://bit.ly/2E8qwSv
- Chanova M, Vuong S, Horak P. Trichobilharzia szidati: The lung phase of migration within avian and mammalian hosts. Parasitol Res. 2007; 100: 1243-1247. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17177058
- Haas W, Pietsch U. Migration of Trichobilharzia ocellata schistosomula in the duck and in the abnormal murine host. Parasitol Res. 1991; 77: 642-644. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1792237
- Chamot E, Toscani L, Rougemont A. Public health importance and risk factors for cercarial dermatitis associated with swimming in Lake Leman at Geneva, Switzerland. Epidemiol Infect. 1998; 120: 305-314. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9692609
- Hoeffler, Dennis F. Cercarial dermatitis: Its etiology, epidemiology, and clinical aspects. Archives of environmental health: An International Journal. 1974; 29: 225-229. http://bit.ly/2rF28oD
- Folster HR, Disko R, Rowert J, Bockeler W, Kreiselmaier I, Christophers E. Cercarial dermatitis contracted via contact with an aquarium: Case report and review. Br J Dermatol. 2001; 145: 638-640. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11703293
- Faltynkova, Anna. Larval trematodes (Digenea) in molluscs from small water bodies near Seske Budsjovice, Czech Republic. Acta Parasitologica. 2005; 50: 49-55. http://bit.ly/35cRvbb
- Faltynkova A, Vanda N, Lenka K. Larval trematodes (Digenea) of planorbid snails (Gastropoda: Pulmonata) in Central Europe: A survey of species and key to their identification. Syst Parasitol. 2008; 69: 155-178. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18210216
- Skirnisson K, L Kolarova. Diversity of bird schistosomes in anseriform birds in Iceland based on egg measurements and egg morphology. Parasitol Res. 2008; 103: 43-50. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18299894
- Ferte H, Depaquit J, Carre S, Villena I, Leger N. Presence of Trichobilharzia szidati in Lymnaea stagnalis and T. franki in Radix auricularia in northeastern France: Molecular evidence. Parasitol Res. 2005; 95: 150-154. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15609060
- Graham, Andrea L. Effects of snail size and age on the prevalence and intensity of avian schistosome infection: Relating laboratory to field studies. J Parasitol. 2003; 89: 458-463. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12880242
- Haas W, Korner M, Hutterer E, Wegner M, Haberl B. Finding and recognition of the snail intermediate hosts by 3 species of echinostome cercariae. Parasitology. 1995; 110: 133-142. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7885732
- Rastyazhenko NM, Vodyanitskaya SN, Yurlova NI. The emission of plagiorchis multiglandularis cercariae from naturally infected snails lymnaea stagnalis in chany lake, South of West Siberia. Parazitologiia. 2014; 49: 190-199. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26373192
- Zbikowska, Elzbieta. Infection of snails with bird schistosomes and the threat of swimmer’s itch in selected polish lakes. Parasitol Res. 2004; 92: 30-35. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14598171
- Loy C, Haas W. Prevalence of cercariae from Lymnaea stagnalis snails in a pond system in Southern Germany. Parasitol Res. 2001; 87: 878-882. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11688896
- McClelland G, Bourns TK. Effects of Trichobilharzia ocellata on growth, reproduction and survival of Lymnaea stagnalis. Exp Parasitol. 1969; 24: 137-146. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/5813415
- Haas W, van de Roemer A. Invasion of the vertebrate skin by cercariae of Trichobilharzia ocellata: Penetration processes and stimulating host signals. Parasitol Res. 1998; 84: 787-795. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9797062
- Grabe K, Haas W. Navigation within host tissues: Cercariae orientate towards dark after penetration. Parasitol Res. 2004; 93: 111-113. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15118907
- Haas W, Haeberlein S. Penetration of cercariae into the living human skin: Schistosoma mansoni vs. Trichobilharzia szidati. Parasitol Res. 2009; 105: 1061-1066. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19543747
- Walter Neuhaus. Biologie und entwicklung von Trichobilharzia szidati n. sp. (Trematoda, Schistosomatidae), einem erreger von dermatitis beim menschen. Parasitology Research. 1952; 15: 203-266. http://bit.ly/2RHglMr
- Jouet D, Ferte H, Hologne C, Kaltenbach ML, Depaquit J. Avian schistosomes in French aquatic birds: A molecular approach. J Helminthol. 2009; 83: 181-189. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19356266
- Jouet D, Kolarova L, Patrelle C, Ferte H, Skirnisson K. Trichobilharzia anseri n. sp. (Schistosomatidae: Digenea), a new visceral species of avian schistosomes isolated from greylag goose (Anser anser L.) in Iceland and France. Infect Genet Evol. 2015; 34: 298-306. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26070888
- Ellis, James C. Host-parasite relationships between Trichobilharzia ocellata and ducks previously exposed to the same parasite. Clin Microbiol Rev. 2015; 28: 165-190. http://bit.ly/35dRX9n
- Horweg C, Sattmann H, Auer H. Cercarial dermatitis in Austria: Questionnaires as useful tools to estimate risk factors?. Wien Klin Wochenschr. 2006; 118: 77-80. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17131245
- Terhune JS, DJ Wise, Lester HK. Bolbophorus confusus infections in channel catfish in northwestern mississippi and effects of water temperature on emergence of cercariae from infected snails. North American Journal of Aquaculture. 2002; 64: 70-74. http://bit.ly/35eJaE4
- Levesque B, Giovenazzo P, Guerrier P, Laverdiere D, Prud HH. Investigation of an outbreak of cercarial dermatitis. Epidemiol Infect. 2002; 129: 379-386. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12403114
- Verbrugge LM, Rainey JJ, Reimink RL, Blankespoor HD. Swimmer's itch: Incidence and risk factors. Am J Public Health. 2004; 94: 738-741. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15117691
- Jekel, Ilse, Zick Daniela. Wasserschnecken und zerkarien im mattsee. Ber Nat med. Ver Salzburg. 2004; 14: 75-93. https://bit.ly/35fO7wq
- Sturm, Robert. Makrofotografie ausgewahlter weichtiere aus heimischen gewassern. Mikrokosmos. 2011; 100: 267.
- Zettler ML. Zur taxonomie und verbreitung der gattung theodoxus montfort, 1810 in deutschland. Darstellung historischer und rezenter daten einschließlich einer bibliografie. Mollusca. 2008; 26: 13-72. http://bit.ly/2tapKCa
- Sluiters JF, Brussaard-Wust CM, Meuleman EA. The relationship between miracidial dose, production of cercariae, and reproductive activity of the host in the combination Trichobilharzia ocellata and Lymnaea stagnalis. Z Parasitenkd. 1980; 63: 13-26. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7415420
- Faltynkova A, Nasincova V, Kablaskova L. Larval trematodes (Digenea) of the great pond snail, Lymnaea stagnalis (L.), (Gastropoda, Pulmonata) in Central Europe: A survey of species and key to their identification. Parasite. 2007; 14: 39-51. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17432056
- Selbach C, Soldanova M, Sures B. Estimating the risk of swimmer's itch in surface waters - A case study from Lake Baldeney, River Ruhr. Int J Hyg Environ Health. 2016; 219: 693-699. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25900402
- Soldanova M, Selbach C, Sures B. The early worm catches the bird? Productivity and patterns of Trichobilharzia szidati cercarial emission from Lymnaea stagnalis. PloS one.2016; 11: e0149678. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26895541

Sign up for Article Alerts