Cytotoxic and Antiviral Activities of Vacuum Liquid Chromatography Fractions of Azadirachta Indica?
Obi RK1,4*, Olayinka OA2, Adesegun SA3, Olaleye DO2 and Omilabu SA4
1Department of Microbiology, Federal University of Technology, Owerri, Imo State, Nigeria
2Department of Virology, College of Medicine, University of Ibadan, Oyo State, Nigeria
3Department of Pharmacognosy, Faculty of Pharmacy, University of Lagos, Nigeria
4Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Nigeria
*Address for Correspondence: Obi RK, Department of Microbiology, Federal University of Technology, Owerri, Imo State, Nigeria, Tel: +234-080-387-57515; ORCID: 000-000-016-524-694X; E-mail: obi.robert@ymail.com
Submitted: 08 February 2020; Approved: 10 March 2020; Published: 18 March 2020
Citation this article: Obi RK, Olayinka OA, Olaleye DO, Adesegun SA, Omilabu SA. Cytotoxic and Antiviral Activities of Vacuum Liquid Chromatography Fractions of Azadirachta Indica. Int J Virol Infect Dis. 2020;5(1): 009-017.
Copyright: © 2020 Obi RK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Viruses; Bioactive compounds; Cellular toxicity; Virucidal inhibition; Adsorption inhibition; Pre-infection inhibition
Download Fulltext PDF
Background: The unavailability of effective therapy and vaccines for some viral infections as well as the rapid emergence of resistance to existing antiviral drugs have called for urgent need to develop new and effective chemotherapeutic agents for viral diseases.
Objective: The aim of this study was to isolate and identify the potential antiviral fractions of Vacuum Liquid Chromatography (VLC) of Dichloromethane (DcM) partitioned fractions of Azadirachta Indica (A. indica) against Measles Virus (MV), Polio Virus (PV), Yellow Fever Virus (YFV) and Herpes Simplex Virus-1 (HSV-1).
Materials and Methods: The fractions were subjected to both cytotoxic and antiviral analyses, as well as Thin Layer Chromatography (TLC).
Results: The results showed that the most toxic VLC fraction of A. indica was 360Hex: 40EAc with IC50 of 0.058 μg/μL, while the least was 160Hex: 240EAc (IC50 = 0.100 μg/μL). Four (66.7%) of the fractions, 360Hex:40EAc, 320Hex:80EAc, 280Hex:120EAc and 200Hex:200EAc, inhibited the viruses, at the same maximum non-toxic concentration (MNTC) of 0.031 μg/μL with YFV being the most susceptible virus to the fractions. The fractions, 160Hex:240EAc and 80Hex:320EAc did not show any observable antiviral activity against any of the viruses. While some of the fractions exhibited only adsorption inhibitory, others were only post-infective, and yet others were both adsorptive and post-infective inhibitory. TLC analysis of the fractions using 8Hex:2EAc solvent system revealed the presence of visible spots with different Retention Factor (Rf) values, identified as bioactive phytochemicals.
Conclusion: This study provides evidence that VLC fractions of A. indica contain medicinally bioactive compounds with observable antiviral activities against MV, PV, YFV and HSV-1.
Introduction
Virus diseases, such as Acquired Immunodeficiency Syndrome (AIDS), poliomyelitis, mumps, measles, herpes, Ebola and yellow fever, are among the leading causes of death worldwide especially in developing countries [1]. Once infected with a virus, little can be done within the limits of contemporary orthodox medicine. Certain antiviral drugs such as interferon, ribavirin, lamivudine, zidovudine and others, may however shorten the progression and course of illness or ameliorate the severity of symptoms, but they are generally expensive and not readily available [2]. Furthermore, currently available antiviral drugs such as acyclovir, like most other drugs, are bedeviled with either severe side effects and/or loss of efficacy due to virus evolution resulting in development of resistance to the drugs [3].
Lack of effective therapy and/or vaccines for several viral infections, and the rapid emergence of drug-resistant viruses have necessitated the need for developing new and effective antiviral agents derived from natural products that are easily accessible for treatment of viral diseases [1]. While there are a few effective antiviral drugs, there are many plant extracts with demonstrable antiviral activities [4], which have, directly or indirectly, been a component of healthcare systems through the ages in all cultures [5]. Advances in the understanding of both the cellular and molecular mechanisms of virus replication have also provided the basis for novel therapeutic strategies. Several hundred natural products have therefore been isolated for identification of antiviral activity, and some have been shown to have great medicinal value in preventing and/or ameliorating virus diseases in preclinical and clinical trials [1]
According to Rodenburg [6], plants are important source of molecules that could be used as a drug. Due to the fact that plant extracts usually occur as a combination of various types of bioactive compounds or phytochemicals with different polarities, their separation is necessary for the process of identification and characterization [7]. The analysis of bioactive compounds present in the plant extracts as reported by Sasidharan, et al. [8] involve the applications of common phytochemical screening assays and chromatographic techniques such as High Performance Liquid Chromatography (HPLC), Column Liquid Chromatography (CLC), Vacuum Liquid Chromatography (VLC), and Thin Layer Chromatography (TLC) to obtain pure compounds.
Indeed botanical and herbal preparations for medicinal usage contain various types of bioactive compounds, which, when extracted and further separated and characterized, could form a basis for the development of new and useful antiviral drugs for treatment of virus infections such as Yellow Fever Virus (YFV), Measles Virus (MV), Poliomyelitis Virus (PV), and Herpes Simplex Virus-1 (HSV-1) that have hitherto defied effective control, either due to none availability and/or lack of affordability of antiviral drugs, viral resistance or adverse reactions to available antiviral drugs.
There are over 20 million reported cases of measles and about 200,000 deaths annually worldwide [9]. Most cases occur in developing countries with weak health infrastructures. Presently, the developing countries including Nigeria account for about 94% of the global deaths caused by measles annually [10,11]. HSV causes herpes, a growing public health problem with high prevalence in women in developing countries, especially in HIV coinfection where incidence of reactivation is high [12]. Polio remains a threat in Nigeria despite the global efforts at eradicating the disease. The residual effects of polio, which has remained a threat to Nigeria, are seen long after the acute phase of the disease has ended, with paralysis lasting for the lifetime of the aff ected individual [14]. Approximately 200,000 cases of yellow fever occur annually, resulting in about 30,000 deaths [13], with nearly 90% of these occurring in the tropical and subtropical areas of South America and sub-Sahara Africa.
Despite the progress made in immunization and drug development, there are still lack of preventive vaccines against many viruses nor efficient antiviral therapies. The available antiviral drugs are often beset by the generation of viral escape mutants. Thus, identifying novel antiviral compounds is of critical importance particularly because natural products have proved to be excellent sources for such discoveries.
Materials and Methods
Study site
Initial tissue culture study was carried out at the Virology Laboratory in the Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos from Jan 2013-Oct 2014. The chromatographic studies were done at the Pharmocognosy Department, School of Pharmacy University of Lagos from Oct 2013-June 2016. Evaluation of antiviral properties of the compounds was done at the Department of Virology, College of Medicine, University of Ibadan from Oct 2014-Oct 2016.
Preparation of plant fraction
Twenty six grams (26g) dichloromethane fraction of A. indica was dissolved in 5 mL of fractionating medium and stirred until slurry was formed. The slurry material was dried to a powdered state. A sintered glass funnel (12 x 7 cm) was packed with 300g silica gel (70-230 mesh) and placed on top of 1L flat bottom flask attached to suction pump. The slurry was then transferred to top of the silica gel and covered with cotton wool. Different proportions of solvent mixtures starting with 100% hexane, hexane-dichloromethane, hexane-ethyl acetate, dichloromethane-methanol and methanol 100% were gradiently introduced, followed by suctioning. Each solvent mixture was collected as separate fraction and concentrated [15] and the fractions analyzed using TLC [16].
Ten mg (1 mg/mL) of each dried solid plant extracts was reconstituted in 0.5% Dimethyl Sulfoxide (DMSO) (Sigma) and brought to a final volume of 10 mL with the addition of 9.95 mL of distilled water. It was filtered with 0.45 µM and 0.22 μM membrane syringe filters (Cell Treat USA). A 100 µL of each fraction concentration was thereafter used for the cellular toxicity evaluation and antiviral assay of the extracts [17].
Evaluation of cellular toxicity
The method used was based on cellular morphologic changes as recommended by Park, et al. [18]. Briefly, Vero cells were prepared at a density of 8 x104 cells mL-1 in a 10% MEM in 75 cm2 tissue culture flasks (Cell Treat, USA). A 100 μL of this cell suspension (containing 8000 cells) was then dispensed into each well of a flat-bottomed 96-well tissue culture plate (Cell Treat, USA) and incubated for 24h at 37°C. The positive control was Virest 200mg, a brand of Acyclovir (Hovid Bhd, Malaysia). The 24h Vero and graded concentrations of the fractions were incubated together in 1% MEM in 96 well tissue culture flasks. Cell viability was monitored daily for 14 days using an inverted microscope (Inverskop 40C) for changes in the morphology, described as Cytopathic Effect (CPE) compared with the uninfected cell control wells containing medium without any extract.
Propagation of test viruses
Measles (Edmonston-Zaghreb strain, Serum Institute, Hadaraba, Pune, India), poliomyelitis (types 1, 2 & 3, Serum Institute, Hadaraba, Pune, India), and yellow fever (17D strain, FSUE of Chimakov IPVE, Russian Acad. Med. Sci.) viruses were propagated from the respective vaccines of the viruses obtained from Institute of Child Health, University College Hospital (UCH), Ibadan. Herpes Simplex Virus-1 (HSV-1) was isolated from the serum of a male patient who presented with HSV-1 symptoms at the Central Research Lab, Lagos University Teaching Hospital (LUTH) and confirmed to be HSV-1 using PCR. Using Reed-Muench method, viral infectivity (TCID50 mL–1) was calculated to be MV 10–1.5, HSV-1 10–3.5, YFV10–4.5, and PV10–4.5 [19].
Virucidal activity
The virucidal or virus inhibitory activity of the compounds was measured by in vitro incubation of viruses with the extracts. Briefly 100µL of (a) virus + extract mixture was inoculated in triplicate unto the 96-well tissue culture plate seeded with Vero cells. Similarly, 100µL of (b) virus + 1% MEM mixture was added in triplicate to the last three wells of each row on the same 96-well tissue culture plate to serve as control. Also added in triplicate were 100µL of acyclovir positive control + 100µL of virus. The last two rows of the wells were used for cell control and extract/fraction control. All the mixtures were incubated at 37°C in 5% CO2 and moisture. The set up was monitored daily under using an inverted microscope (Inverskop 40C) for 7 days and scored for virus-induced CPE [19].
Adsorption/ post adsorption assays: A hundred microliter (100 μL) of Vero cell line was added to each of 96-well of a microtiter plate and incubated with 100 μL of different concentrations of each plant extract at 37°C for 2hr in a 5% CO2. The extracts were removed and washed with PBS and cell monolayers were infected with 100 μL of 100 TCID50 of each virus dilution. This was incubated for 7 days and monitored for CPE using an inverted microscope (Inverskop 40C) and scored (25). A Hundred microliter of 100 TCID50 of each virus dilution in 1% MEM medium was added to Vero cell monolayer and incubated in a 5% CO2 incubator for 2hr. The viral inoculum was aspirated and discarded and cell monolayer washed with PBS, refreshed with 1% MEM medium containing different extract concentrations and incubated at 37°C in a 5% CO2 incubator and the presentation of CPE was investigated daily for 7 days using an inverted microscope (Inverskop 40C) and scored for appearance, nature and degree of CPE [19].
Statistical analyses
Results were presented as standard error of mean (SEM). Data were assessed by one-way analysis of variance (ANOVA) followed by Duncan multiple comparison, Turkey’s multiple comparison and student’s t-test. All statistical analysis were performed at the p < 0.05 level of significance. All the statistical analysis were done using GraphPad software version 5.01 (GraphPad Software Incorporated, U.S.A, 2007).
Results
Thin layer chromatography
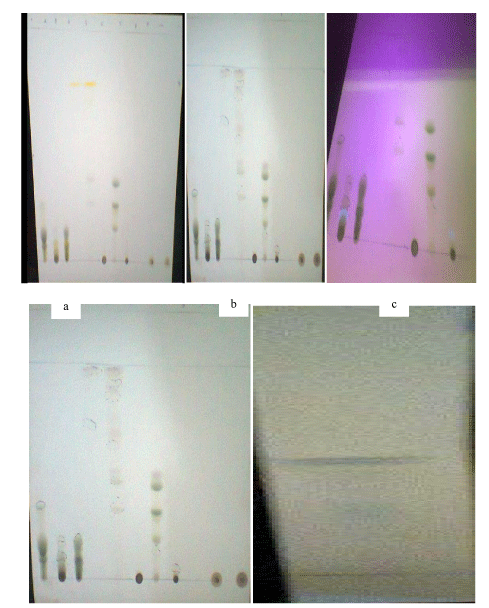
The outcomes of characterization of the vacuum liquid chromatography fractions of Azadirachta Indica showed the presence of compounds representated by visible spots and their retention factor values. Table 1 showed that at the Rf value of 0.05 the fraction 200Hex:200EAc produced a yellow colour in daylight, and a green colour at 254 nm UV light, 366 nm UV light and after spray with 10% sulphuric acid. The fraction 340Hex:60EAc produced yellow colour in daylight, green colour at 254 nm UV light, blue colour at 366 nm UV light, and a brown colour after spray with 10% sulphuric acid at the Rf value of 0.99. Plate 1 shows the colours of the spots in daylight, 254nm and 366nm, and after spraying with 10% H2SO4.
Cytotoxicity analysis
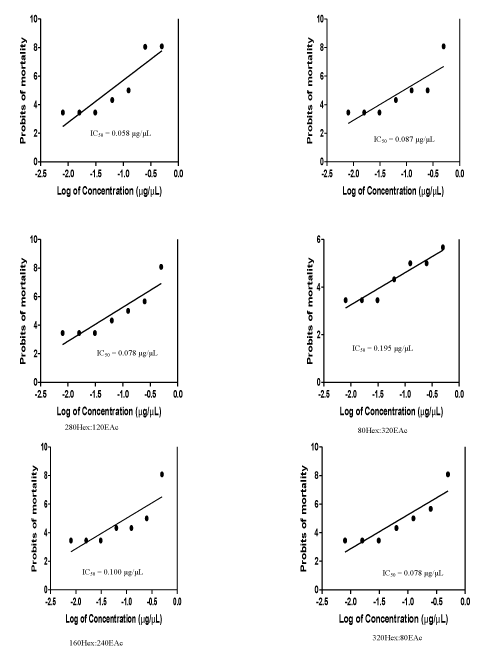
Result of the toxicity analysis illustrated in figure 1 showed that the most toxic of the VLC fractions was 360Hex:40EAc with IC50 = 0.058 μg/μL, while the least was 160Hex:240EAc (IC50 = 0.100 μg/μL). The Maximum Non-Toxic Concentration (MNTC) of all the fractions, which was 0.031 μg/μL, was lower than all the IC50 of the fractions.
Virucidal analysis
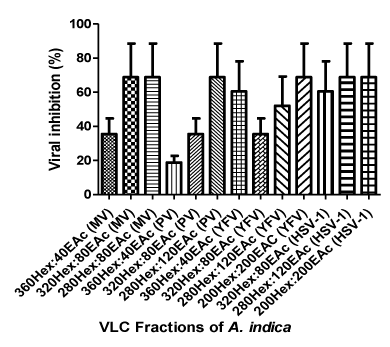
The antiviral activities of the VLC fraction are presented in tables 2-5 and indicated that the most potent fractions were 320Hex:80EAc and 280Hex:120EAc. These fractions inhibited all the test viruses. The fractions, 160Hex:240EAc and 80Hex:320EAc did not show any observable antiviral activity against any of the viruses. The percentage inhibitory activity of the fractions on the viruses (Figure 2) showed that the most susceptible virus was HSV-1 (60.4%) while the least was Poliovirus with 35.5%.
Adsorption and post adsorption inhibition assays
The result in (Table 6) showed that the fractions exhibited both adsorption and post-infection antiviral activities on the viruses. While some of the fractions, 360Hex:40EAc and 320Hex:80EAc exhibited only adsorption inhibitory activity on MV, others, 280Hex:120EAc were only post-infective also on MV. In PV infection 360Hex:40EAc and 280Hex:120EAc were post infection inhibitory while 320Hex:120EAc maintained its adsorption inhibition. In YFV infection, 360Hex:40EAc and 320Hex:80EAc were both adsorption and post infection inhibitory. In HSV-1 infection, 360Hex:40EAc and 280Hex:120EAc inhibited the virus in both adsorption and post infection assays.
Selectivity index
The result of the Selectivity Index (SI) shown in table 7 revealed that the IC50 of the fractions was far less than the calculated SI that inhibited the test viruses, indicating that they could be good candidates for drug production against the viruses. The highest selectivity index of 39.0 was produced by 320Hex:80EAc and 280Hex:120EAc on Poliovirus. The same fractions also produced the least SI 2.05 on HSV-1.
Discussion
The unavailability and unaffordability of most of the available antiviral drugs and emergence of multi-drug resistance in viruses have, indeed, triggered immense interest in the search for new antiviral agents of plant origin [1]. Specific events in virus replication steps identified as targets for antiviral agents include viral adsorption, penetration, uncoating, viral nucleic acid and protein syntheses [20].
In this study it was observed that all the fractions inhibited proliferation of Vero cells at IC50 values below < 20 µg/mL as recommended by the National Cancer Institute (NCI) for crude extracts [21]. According to this standard, any crude extract with IC50 values below < 20 µg/mL, could be considered safe to be applied, directly to mammalian cells as drugs, or indirectly, as pharmaceutical raw materials for antiviral drug production.
The antiviral activities of the extracts were analyzed using three RNA test viruses, namely, Measles, Polio, Yellow fever; and one DNA virus, Herpes Simplex-1 (HSV-1). The result showed broad spectrum antiviral activities against the viruses with 320Hex:80EAc inhibiting replication of all the viruses. Possession of viral envelop, capsid or type of nucleic acid, did not confer any form of special protection on the viruses, since both RNA and DNA, enveloped and non-enveloped were, affected. The pre- and post- infection inhibitory assays (Table 6) revealed that the viruses showed sensitivity to the fractions at both the adsorption and post- infection inhibitory assays indicating that, these fractions, if properly harnessed, could become pharmaceutical raw materials for drug development, for the prevention and/or treatment of infections due to susceptible viruses. These two fractions, 320Hex:80EAc and 280Hex:120EAc, may have inhibited the viruses due to the presence of bioactive compounds and this confirms the findings of earlier studies that bioactive plant components are responsible for their medicinal properties [8,22]
The colours of the spots observed in daylight, UV and after spraying with 10% H2SO4, in the TLC analysis of A. indica were indicative of the presence of bioactive secondary metabolites in the plant. The colours (Table 1 and Plate 1): black, grey, green, yellow, brown, dark blue, and blue, signified the presence of terpenoids, cardiac glycosides, steroids, alkaloids, carbohydrates, phenolic compounds, and glucose, respectively. The presence of a fluorescent spot on the 360nm UV lamp was indicative of the presence of aromatic and polycyclic compounds [23]. These bioactive phytochemicals, which have variously been reported as antimicrobials [24], might have been responsible for the antiviral activities in the fractions tested in this study.
Useful antiviral compounds must differentiate between host and viral functions with a high degree of specificity, must be highly selective and specific, inhibiting one or more steps of virus replication without causing unpleasant side effects [20]. The clinical value of the compounds used in this study was determined by their Selectivity Index (SI). All the fractions had SI values that exceeded their IC50 values. As reported by De Clercq [25], a compound with a low IC50 and a high SI is most likely to have value as an antiviral drug. As a result therefore all the fractions which showed inhibitory activities against the viruses used in this study could become the basis for antiviral drug development and could safely be administered for the prevention and treatment of infections caused by the viruses.
Conclusion
This study has shown that herbal preparations contain various types of bioactive compounds, which could form a good basis for the development of new and useful antiviral drugs for the prevention and treatment of those highly virulent viruses that have hitherto been very difficult to control with orthodox drugs particularly due to rapid occurrence of drug resistant virus strains. Although much work has been done with all parts of A. indica, this work is significant, especially as this could be the first time a study was conducted to challenge the efficacy of the plant on such viruses as HSV-1, PV and YFV.
Significance of the Study
The plant used in this study was selected based on history of its medicinal and anti-infective properties, as reported in the ethnobotanical survey of Nigerian medicinal plants and discussion with herbalists and herb sellers. The study then, through preliminary studies, confirmed its medicinal properties as well as potential antiviral activities against common viruses of public health significance in Nigeria, which have hitherto defied successful treatment with orthodox drugs. This study will therefore be of immense benefit to traditional medicine practitioners and rural dwellers who consume this medicinal plant and rely on it for their healthcare needs. In addition, it is hoped that the findings from this study will be used by reputable pharmaceutical companies (local and foreign) as raw materials for drug production against susceptible viruses.
- Kitazato K, Wang Y, Kobayashi N. Viral infectious diseases and natural products with antiviral activity. Drug Discov Ther. 2007; 1: 14-22. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22504360
- Song JM. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005; 68: 66-74. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16137775
- Strasfeld L, Chou S. Antiviral drug resistance: Mechanisms and clinical implications. Infect Dis Clin North Am. 2010; 24: 413-437. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20466277
- Davies J. Anti-viral activity of plant extracts and herbs. International Journal of Tropical Medicine. 2003; 2: 49-54.
- Falodun A. Herbal Medicine in Africa-distribution, standardization and prospects. Research Journal of Phytochemistry.2010; 4: 154-161. http://bit.ly/38AtgEY
- Rodenburg R. Screening for health effects of herbs In Handbook of herbs and Spices. England: Woodhead Publishing Limited; Abington Hall, Abington Cambridge CB1 6AH. p. 54-65.
- Eberhardt TL, Li X, Shupe TF, Hse CY. Chinese tallow tree (Sapium Sebiferum) utilization: Characterization of extractives and cell-wall chemistry. Wood Fiber Science. 2007; 39: 319-324. http://bit.ly/2TTxpOJ
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga LL. Extraction, isolation and characterization of bioactive compounds from plants' extracts. African Journal Traditional and Complement Alternative Medicine. 2011; 8: 1-10. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22238476
- WHO/UNICEF Joint Statement. Reducing measles mortality in emergencies. World Health Organization. 2012. http://bit.ly/2U2JUaL
- Griffin DE, Knipe DM, Howley PM, Lamb RA, Martin MA, et al. Measles virus. In Fields Virology, 4th edition. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1401-1441.
- Strauss JH, Strauss EG. Viruses and human disease, 2nd edition. Academic Press, Burlington, USA. 2008. p. 147-158
- Mina Kalantari-Dehaghi, Sookhee Chun, Aziz Alami Chentoufi, Jozelyn Pablo, Li Liang, Gargi Dasgupta, et al. Discovery of Potential Diagnostic and vaccine antigens in herpes simplex virus 1 and 2 by Proteome-Wide antibody profiling. Journal of Virology. 2012; 86:4328-4339. http://bit.ly/2TKkNuA
- WHO. Vaccines and vaccination against yellow fever. WHO position paper-June 2013. Weeekly Epidemiological Records. 2013; 88: 269-283. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24852721
- Weekly epidemiological report on poliomyelitis. NCDC. 2017. http://bit.ly/2Wb3vZ9
- Noor-Ul-Amin, M Imran Qadir, Tahir Javed Khan, Ghulam Abbas, Bashir Ahmad, Khalid Hussain Janbaz, et al. Antibacterial Activity of Vacuum Liquid Chromatography (VLC) Isolated Fractions of Chloroform Extracts of Seeds of Achyranthes Aspera. Journal of Chemical Society of Pakistan. 2012; 34: 589. http://bit.ly/39NBZoK
- Velmurugan S, Babu MM, Punitha SMJ, Viji VT, Citarasu T. Screening and characterization of antiviral compounds from Psidium guajava Linn. Root bark against white spot syndrome virus. Indian Journal of Natural Products and Resources. 2012; 3: 208-214. http://bit.ly/2PGjSsT
- Beltran OMS. Investigation of the anti-mycobacterial and cytotoxic effect of three medicinal plants used in the traditional treatment of tuberculosis in northern Mexico and the Southwest United States. 2008. http://bit.ly/2W5P1tF
- Park IW, Han C, Song X, Green LA, Wang T, Liu Y, et al. Inhibition of HIV-1 entery by extracts derived from Chinese medicinal herbal plants. BMC Complement Altern Med. 2009; 9: 29. PubMed:https://www.ncbi.nlm.nih.gov/pubmed/19656383
- Park IW, Han C, Song X, Green LA, Wang T, Liu Y, et al. Antiviral effect of Hibiscus sabdariffa and Celosia argentea on measles virus. African Journal of Microbiology Research. 2010; 4: 293-296. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19656383
- De Clercq E, Field HJ. Antiviral prodrugs-the development of successful prodrug strategies for antiviral chemotherapy. Br J Pharmacol. 2006; 147: 1-11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16284630
- Abdel-Hameed ES, Salih A Bazaid, SA Shohayeb, MM El-Sayed, MM, El-Wakil EA. Phyto chemical studies and evaluation of antioxidant, anticancer and antimicrobial Properties of Conocarpus erectus L. Growing in Taif, Saudi Arabia. European Journal of Medicinal Plants. 2012; 2: 93-112. http://bit.ly/2wSbX51
- Chikezie PC, Ibegbulem CO, Mbagwu FN. Biactive principles from medicinal plants. Research Journal of Phytochemistry. 2015; 9: 88-115. http://bit.ly/2TYiOlj
- Kumar S, Iyotirmayee K, Sarangi M. Thin layer chromatography: A tool of Biotechnology for isolation of bioactive compounds from medicinal plants. International Journal of Pharmaceutical Science Reviews and Research.2013; 18: 126-132. http://bit.ly/2wN7sIW
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999; 12: 564-582. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10515903
- De Clercq E. Antiviral drug discovery and development: Where chemistry meets with biomedicine. Antiviral Research. 2005; 67: 56-75. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16046240

Sign up for Article Alerts