Risk Factors and Seroepidemiologic Analyses of Herpes Simplex Virus Types 1 and 2 in Assymptomatic Persons in a Tertiary Institution in South Eartern Nigeria?
Obi RK1*, Shenge JA2, Chikwendu CI1 and Amadi ES1
1Department of Microbiology, School of Biological Sciences, Federal University of Technology, Owerri, Imo State, Nigeria
2Virology Unit, Department of Biological Sciences, College of Science, Dominican University, Ibadan, Oyo State, Nigeria
*Address for Correspondence: Obi RK, Department of Microbiology, Federal University of Technology, Owerri, Imo State, Nigeria, Tel: +234-080-387-57515; ORCID: 000-000-016-524-694X; E-mail: obi.robert@ymail.com
Submitted: 08 February 2020; Approved: 10 March 2020; Published: 18 March 2020
Citation this article: Obi RK, Chikwendu CI, Amadi ES, Shenge JA. Risk Factors and Seroepidemiologic Analyses of Herpes Simplex Virus Types 1 and 2 in Assymptomatic Persons in a Tertiary Institution in South Eartern Nigeria. Int J Virol Infect Dis. 2020;5(1): 018-023.
Copyright: © 2020 Obi RK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: ELISA kit; FUTO; Students; Blood sample; IgG antibody; Risk factors
Download Fulltext PDF
Background: Herpes simplex virus infection is a global public health concern with disproportionately high burden in low and middle - income countries. There is paucity of data on the prevalence of HSV-1 and HSV-2 infection among undergraduates of tertiary institutions in South Eastern, Nigeria. This prompted the present study.
Aim: The aim of the study was to provide an up-to-date data on sero-prevalence, as well as risk factors of HSV-1 and HSV-2 infections among undergraduate students of a tertiary institution in South Eastern Nigeria.
Materials and Methods: A structured questionnaire was administered to each of the study participants to obtain information on the possible indulgence of the risk factors that could predispose to HSV. Blood samples were collected from 200 male and 200 female consenting undergraduate students aged between 15 and 32. Sera were analyzed for HSV-1 and HSV-2 antibody using ELISA IgG Antibody kit (DIA. PRO Diagnostic Bioprobes, Italy).
Results: Results showed that the age group with the highest prevalence of HSV-1 antibody was 21-23 years with 33.8% while the least was 30-32 with 5.8%. The age group with the highest prevalence of HSV-2 antibody was 24-26 with 40%, while the least were 15-17 and 30-32 with no antibody response to the virus. The sex distribution of HSV-1 and HSV-2 infection showed that both infections were more prevalent in females than in males with prevalence of 61.3% and 37% respectively for HSV-1 and 13.2% and 11.3% respectively for HSV-2. The result of the coinfection of HSV-1 and HSV-2 showed that the age interval of 24-26 showed more antibody response (40%) than the other age groups. The results also showed that the highest predisposing risk factors for HSV-infection was exposure to sunlight (99.6%) followed by stress (99.3%), then multiple sex partners (85.2%) and indulgence in oral sex (17.2%).
Conclusion: The results highlight the potential public health impact of HSV-1 and HSV-2 in Nigeria especially among young adults, where anti-HSV-1/2 testing is not generally performed in all populations, hence remain a neglected disease.
Introduction
Herpes Simplex Virus (HSV) infection is a common cause of ulcerative mucocutaneous disease in both immunocompetent and immunocompromised individuals. It is a growing worldwide public health problem with high prevalence in women in developing countries, especially those with HIV infection, where incidence of reactivation is high [1].
Herpes simplex viruses are categorized into two types: Herpes type 1 (HSV-1 or oral herpes) and herpes type 2 (HSV-2 or genital herpes). Oral and ocular lesions are primarily caused by HSV-1 and genital lesions by HSV-2. However, HSV-2 is capable of being transmitted to newborns during peripartum and cause ocular lesions as a result of disrupted membranes, or by direct contact with infected mother’s vaginal secretions and infected cervix [2]. These viruses are also capable of causing more serious diseases, such as blindness, meningitis, and encephalitis [3]. HSV-1 is recognized as a leading cause of viral corneal blindness and viral encephalitis in developed countries [4].
The mature infectious HSV consists of four components from the core outward including an opaque dense core that contains linear double stranded DNA approximately 152 kbp; encoding at least 74 genes or Open Reading Frames (ORFs). The genome is complex and contains two unique regions called the Long Unique Region (UL) and the Short Unique Region (US). Of the 74 known ORFs, UL contains 56 viral genes, whereas US contains only 12 [5].
HSV-1 is often acquired orally during childhood [6]. It may also be sexually transmitted, contracted through saliva as well as kissing and oral sex. HSV-2 is primarily a sexually transmitted infection but rates of genitally acquired HSV-1 are also increasing [7]. Both viruses may also be transmitted vertically during childbirth, although the risk is very low [8]. However the risk of infection is minimal if the mother has no symptoms or exposed blisters during delivery but considerable when the mother gets the virus for the first time during late pregnancy [6].
Worldwide, approximately 90% of people are infected with HSV-1 or HSV-2 or both. HSV-1 is the more prevalent virus, with more than 3.7 billion people under the age of 50 (67%) infected globally. In addition, about 140 million people aged 15-49 years are infected with genital HSV-1 infection. Estimates of new HSV-1 infections among people aged 0-49, showed that in Africa, there were 17 million women and 18 million men living with the virus by December, 2012. In people with weak immune systems, such as those with advanced HIV infection, HSV-1 can have more severe symptoms and more frequent recurrences [9]. As of 2012, HSV-2 was estimated to infect 417 million people worldwide between the ages of 15 to 49 years, giving a global prevalence of 11.3%, with 19.2 million infections each year. HSV-2 prevalence is nearly 2-fold higher in women than in men (14.8% versus 8% global prevalence, respectively) [10].
There is no cure for HSV. Antiviral medications, acyclovir (Zovirax), famciclovir (Famvir), and valacyclovir (Valtrex), commonly prescribed for treatment of HSV only help lesions to heal faster during an initial outbreak, lessen the frequency and duration of symptoms during recurrences, reduce the frequency of outbreaks, and decrease viral shedding [11]. Systemic antiviral drugs can partially control the signs and symptoms of genital herpes when used to treat first clinical and recurrent episodes or when used as daily suppressive therapy [12]. Use of pericoital tenofovir gel by women prior to sexual intercourse also serves to prevent transmission of herpes [13].
Materials and Methods
Study design
The study involved a serological analysis of samples, using an ELISA kit (DIA PRO Diagnostic Bioprobes, Italy) to determine and quantify herpes simplex virus types 1 and 2 antibodies among selected students of the Federal University of Technology Owerri, Imo State, Nigeria.
Study population
The study population included apparently healthy 200 male and 200 female undergraduate students of Federal University of Technology, Owerri, selected at random. Their ages ranged between 15 and 32 years. The purpose of the study was fully explained to them and their informed consents obtained before sample collection.
Ethical consideration
Ethical approval for the study was obtained from the Medical Ethical and Scientific Research Committee of the School of Biological Sciences, Federal university of Technology, Owerri, before the study commenced.
Inclusion/exclusion criteria
This includes all consenting students within the age range of 15-32, who are undergraduates of Federal University of Technology, Owerri. Students below the age of 15 years, those above the age of 32, and those within the stipulated age range, who did not give their consent were excluded in the study.
Administration of questionnaire
A 16-item structured questionnaire was distributed to the participating students to obtain information on their socio-demographic, clinical data, and possible indulgence on the potential risk factors that might be associated with HSV 1 and 2. The identities of the students were protected by assigning numbers to them and information obtained was treated with utmost privacy.
Sample size determination
The sample size was determined using the equation by Naing, et al. [14] and a reported 44.3% prevalence of HSV-2 infection among pregnant women attending antenatal clinics in Benin, Edo State, Nigeria [15] at 95% confidence interval.
Where n = no of samples; p = prevalence rate of previous study = 44.3%; z = standard normal distribution at 95% confidence limit = 1.96; d = absolute desired precision of 5% = 0.05; q = 1-p = 1-0.443 = 0.557; n = (1.96)2 x 0.443 x 0.557/ (0.952 =379.17). The calculated sample size was 379.17. Therefore 400 blood samples were collected from the students of FUTO.
Sample collection
A 5 mL venous blood sample was obtained from each of the 400 participating students using a sterile disposable hypodermic syringe. The blood samples were appropriately labeled according to the code on the volunteer questionnaire and were taken immediately to the laboratory of the Department of Microbiology, Federal university of Technology, Owerri, for analysis. The samples were transferred into sterile 15 mL centrifuge tubes and allowed to stand for 1 hour to allow clotting. The samples were then centrifuged at 2000rpm for 2 minutes. The supernatant was decanted into sterile screw cap tubes and stored at -20°C till further analysis.
HSV-1 and 2 IgG antibodies
The serum samples were screened for the presence of HSV-1 and 2 IgG antibodies using the ELISA kit, DIA PRO Diagnostic Bioprobes, Italy. The analysis was carried out according to the manufacturer’s instructions. Check value of the control or patient sample / extinction value of calibrator 3 = Ratio. The results were interpreted as described by the manufacturers’ kit insert.
Ratio <0.07: was considered negative
Ratio ≥0.07 to <0.09: was considered borderline
Ratio ≥1.1: was considered positive
Results
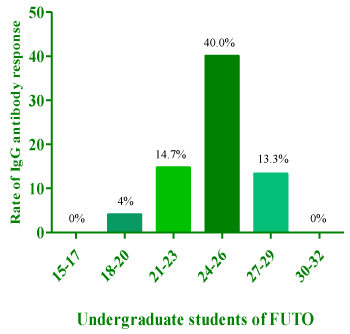
This is a study on the seroprevalence of Herpes Simplex Virus type 1 and 2 among apparently healthy asymptomatic students of Federal University of Technology, Owerri. Table 1 represents the result of the age distribution of the Respondents. A total of 393 out of 400 (98.5%) showed IgG antibody response to HSV-1. The age interval of 21-23 showed the highest response with 135 (33.8%) students, while the least was produced by the age group 30-32 with 23 (5.8%). Table 2 shows the result of the age distribution of the respondents to HSV-2 IgG antibody. A total of 48 respondents (72%) showed positive IgG antibody response to HSV-2. The age group of 24-26 showed the highest response with 20 (40%) while the least came from the age groups of 15-17 and 30-32 which showed no IgG antibody response to the virus.
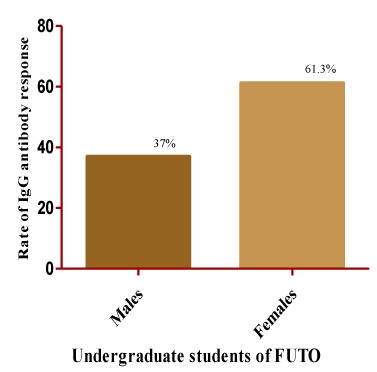
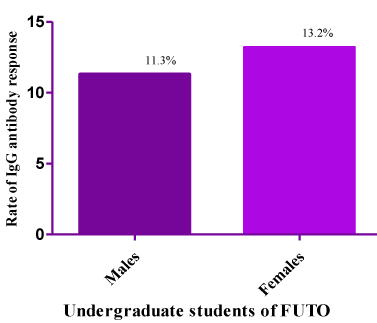
The respondents were also screened according to sex. In figure 1 female respondents showed more IgG antibody response of 61.3% than the males (37%). In figure 2, it was observed that the rate of response to IgG antibody to HSV-2 was 13.2% among the female respondents while it was 11.3% for the males. The result in figure 3 showed the percentage of the students with IgG antibody response to both HSV-1 and 2. The result showed the age interval of 24-26 as the highest response of 40%. The age group of 18-20 showed a response of 4%, while the age group of 15-17 and 30-32 did not show any response. When a combined infection of HSV 1/2 was determined, it was discovered that the age group of 24-26 showed the highest response to both viruses as shown in figure 3. The assessment of risk factors showed that exposure to intense sunlight (99.6%) was the major predisposing factor to HSV-1 infection while possession of multiple sex partners (85.2) was the major factor contributing to HSV-2 infection (Table 3).
Discussion
Infection with HSV-1 and HSV-2 are among the most common human viral infections. While HSV-1 is the cause of orolabial herpes; HSV-2 is the cause of genital herpes. However, due to individual’s sexual behaviours, HSV-1 can now cause genital herpes, while HSV-2 can cause orolabial herpes as well. About 70% genital herpes are not recognized thereby making seroepidemiological surveys a necessity, to understand the pattern and distribution of HSV infection within the population [16].
The prevalence of HSV-1 in FUTO was 98.5% while it was 72.0% for HSV-2. The high prevalence of HSV-1/2 may have been observed due to exposure of the respondents to the antibody against the virus early in life. In addition, HSV-2 is sexually transmitted and as such is a good marker of sexual behavior in the study population. The high prevalence of HSV-1 in comparison to HSV-2 observed in this study is in agreement with that made in previous studies, 63.6% (HSV-1), 36.4% (HSV-2) in Nigeria (16); 99.2% (HSV-1), 78.4% (HSV-2) in Ghana (19); 67% (HSV-1), 11.3% (HSV-2) global (9). This study has further confirmed that HSV-1 infection is universal and usually acquired from intimate contact with family in early childhood [17]. Furthermore, the varying prevalence rate of HSV-2 across the world might be due to changing sexual behaviors among the sexually active population and the ease of vertical transmission of the virus from mother to child [16].
The age group, 21-23 appeared to show more response (33.8%) to the antibody against HSV-1 than the other age intervals. This may be due to the fact that this age group is more exposed to the virus more than the other age groups, probably through intimate family contact at early childhood and also indulgence in oral sex. The presence of HSV-1 antibody in all the age groups is an indication of the universality of this infection and the fact that people acquire the infection in childhood through oral-to-oral transmission from family members. The findings in this study corroborates that of Thahiru, et al. [16] in Abuja, Nigeria where HSV-1 antibody was observed in all age groups with the age group of 21 and above showing the greatest response at 14.3%. A similar finding was also reported by Harfouche, et al. [18] in other parts of Africa. However, the study did not agree with that of Oksana, et al. [19] in Ghana where this age group showed the least response (3.2%), although antibody was prevalent in all age groups. Antibody response to HSV-1 infection followed the pattern of the other responses observed in other parts of the world, where responses decreased with increase in age.
The age group of 21-24 showed more response to antibody against HSV-2 at 40.0% than the other age groups. The major means of transmission of HSV-2 is sexual intercourse and it is possible the infection is more prevalent in this age group because they are exposed to unprotected sex and at the highest sexual activity also than the other groups. The age group of 15-17 and 30-32 did not show any response probably because these groups are more restricted in their sexual behaviours than the other age groups. This study corroborates that of Oksana, et al. [19] and Thahiru, et al. [16], but disagreed with most others across Nigeria where the highest response of IgG antibody to HSV-2 are observed in other age groups [20-22]. This study also disagreed with the popular assertion that the prevalence of HSV-2 infection increases with age since the age group of 30-32 did not show any antibody response to the infection. Although latent infection is common with HSV.
The pattern of HSV-1/2 response in FUTO followed similar patterns across the world, where infection is more prevalent in females than in males. This may be as a result of the fact that females indulged more in the risk factors that predispose to both infections than their male counterparts. Furthermore, studies have revealed that females mount more vigorous immune responses, especially humoral responses to some viral infections [23]. This may have contributed in no small way in accounting for the increase in more females showing more responses to IgG antibody to HSV-1/2 than males in this study.
Our findings that the young age group of 15-17 were HSV-1 positive but HSV-2 negative probably because, prior HSV-1 infection partially protects and delays acquisition of HSV-2. This may also explain the high prevalence of HSV-1 at all ages in comparison to HSV-2. There is therefore a strong evidence in this study to suggest that, prior HSV-1 infection increased the likelihood that an infection that an infection with HSV-2 could be subclinical. This is in direct agreement with previous studies [24-26], all of which confirmed that prior infection with HSV-1 may provide partial protection against infection with HSV-2. However, this study did not agree with the findings of some other workers [27,28] that prior infection with HSV-2 could prevent infection with HSV-1 since the rate of the latter infection was high even in the presence of HSV-2 infection.
After primary infection, further infections of HSV-1 and HSV-2 are `provoked by fever, viral infection, stress, menstrual cycles and perhaps bright sunlight [29]. While exposure to bright sunlight accounted for most of the infections of HSV-1 among the study population, possession of multiple sex partners was the major risk factor responsible for infection of HSV-2. Most of the study subjects must have therefore acquired HSV-1 infection in early childhood which was reactivated by exposure to intense sunlight. It is also confirmed in this study that HSV-2 is purely a sexually transmitted infection. In addition, while stress was the next major factor contributing to HSV-1 reactivation, indulgence in oral sex was second greatest risk factor accounting for HSV-2 infection. Stress lowers the immune system thereby encouraging the reactivation of HSV-1 infection. Stress was also found to be the major risk factor responsible for high prevalence of HSV-1 infection among a population of university students in Germany and Spain [30]. For HSV-2 to be transmitted by oral sex show that the virus was previously present in the mouth thereby suggesting that HSV-2 could be responsible for oral herpes. The finding in this study that 97.9% and 8.2% of the respondents were positive for HSV-1 and HSV-2 respectively although they claimed not to have heard of either infection, was quite alarming and would contribute in small way in making them difficult to control.
Conclusion
The prevalence of HSV-1 and HSV-2 among undergraduate students of Federal University of Technology, Owerri, Nigeria, was very high. All the major risk factors that have been documented to predispose to HSV were implicated in the high prevalence of both infections. It was also observed that quite a number of the study participants had neither heard of HSV-1 or HSV-2. This could be due to the lack of awareness of some viral infections among the population. There is therefore, the need to raise awareness through organized public health screening and education to ensure control.
- Kalantari-Dehaghi M, Chun S, Chentoufi AA, Pablo J, Liang L, Dasgupta G, et al. Discovery of Potential Diagnostic and Vaccine Antigens in Herpes Simplex Virus 1 and 2 by Proteome-Wide Antibody Profiling. J Virol. 2012; 86:4328-4339. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22318154
- Taylor S. Herpes simplex virus. 2017. http://bit.ly/33dp0Kx
- Connolly SA, Jackson JA, Jadetsky TS, Longnecker R. Fusing structure and function: A structural view of the herpes virus entry machinery. Nat Rev Microbiol. 2011; 9: 369-381. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21478902
- Shoji H, Azuma K, Nishimura Y, Fujimoto H, Sugita Y, Eizuru Y. Acute viral encephalitis: The recent progress. Intern Med. 2002; 41: 420-428. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12135172
- McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006; 117: 90-104. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16490275
- Kimberlin DW, Prober GC. Pathogenesis and diseases. In Human herpes viruses:Bology, therapy and immunoprophylaxis. Arvin A, Campdelli-Fiume G, Mocarski E (Editors). Cambridge University Press. 2007; 256-257. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21348130
- Gupta R, Warren T, Wald A. Genital herpes. Lancet.2007; 370: 2127-2137. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18156035
- Corey L, Wald A. Maternal and Neonatal HSV Infections. N Engl J Med.2009; 36: 1376-1385. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19797284
- WHO. Globally, an estimated two-thirds of the population under 50 are infected with herpes simplex virus type 1. 2015. http://bit.ly/33hCapz
- Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015; 10: e114989. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25608026
- Haldiman KL. The use of herbal and dietary supplements in the treatment of herpes simplex virus. Herpes Herbal. 2014; 1-4. http://bit.ly/2xIyjX5
- Leung DT, Sacks SL. Current treatment options to prevent perinatal transmission of herpes simplex virus. Expert Opin Pharmacother.2003; 4: 1809-1819. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14521490
- Salim SA, Quarraisha AK, Kharsany AB, Baxter C, Grobler AC, Werner L, et al. Tenofovir gel for prevention of herpes simplex virus type 2 infection. New England Journal of Medicine. 2015; 373: 530-539. http://bit.ly/2IQYUDy
- Naing L, Winn T, Rushi BN. Practical issues in calculating the sample size for prevalence studies. Arch Orof Sci. 2006; 1: 9-14. http://bit.ly/2QzlMvR
- Kalu EI, Ojide CK, Chuku A, Chukwuonye II, Agwu FE, Nwadike VU, et al. Obstetric outcomes of human herpes virus-2 infection among pregnant women in Benin, Nigeria. Niger J Clin Pract.2013; 18: 453-456. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25966714
- Thahiru Y, Haruna SA, Garba HZ. Seroprevalence of herpes simplex virus among human immunodeficiency virus-positive patients in resource poor settings. Journal of Global Infectious Disease. 2019: 11: 107-111. http://bit.ly/2Qe6j43
- Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al. Pathogenesis and disease, in Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press. 2007; 32: 645-667. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21348071
- Harfouchea M, Hiam C, Laith JA. Herpes simplex virus type1 epidemiology in Africa: Systematic review, meta-analyses, and meta-regressions. Journal of Infection. 2019; 13: 48. http://bit.ly/3cZWcJL
- Debrah O, Agyemang-Yeboah F, Asmah RH, Timmy-Donkoh E, Seini MM, Fondjo LA, Sight N, et al. Seroprevalence of herpes simplex virus type 1 and 2 among women attending routine cervicare clinic in Ghana. BMC Infectious Diseases. 2018; 18: 378. https://www.ncbi.nlm.nih.gov/pubmed/30086705
- Ameh RE, Aminu M, Ella EE. Seroprevalence of HSV-2 among women of reproductive age in Zaria, Kaduna State. Biology and Medicine. 2016; 8: 1-6. http://bit.ly/33nY6PS
- Agabi YA, Banwat EB, Mawak JD, Lar PM, Dashe N, Dashen MM, et al. Seroprevalence of herpes simplex virus type 2 among patients attending sexually transmitted disease infections clinic in Jos, Nigeria. The Journal of infection in developing countries. 2010; 4: 572-575. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21045370
- Mathew O, Ndomb T, Onakewhor J, Matawals B, Osagiei E. Multi-center study on prevalence of human immunodeffiency virus type-2 co-infection among pregnant women in Nigeria. International Journal of Virology and AIDS. 2019; 6: 6-7. http://bit.ly/2TQYXpf
- Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, Cantin E. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon mutant mice. Journal of Virology. 2001; 75: 3048-3052. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11222734
- Breinig MK, Kingsley LA, Armstrong JA, Freeman DJ, Ho M. Epidemiology of genital herpes in Pittsburg: Serologic, sexual, and social correlates of apparent and inapparent herpes simplex infections. J Infect Dis. 1990; 162: 299-305. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2165102
- Koutsky LA, Stevens CE, Holmes KK, Ashley RL, Kiviat NB, Critchlow CW, et al. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med. 1992; 326: 1533-1539. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1315930
- Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study group. N Engl J Med.1999; 341: 1432-1438. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10547406
- Nahmias AJ, Lee FK, Beckman-Nahmias S. Seroepidemiological and sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl.1990; 69: 19-36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2175939
- Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med.1997; 337: 509-515. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9262493
- Pebody RG1, Andrews N, Brown D, Gopal R, De Melker H, François G, et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. 2004; 80: 185-191. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15170000
- Stock C, Guillen-Grima F, de Mendoza JH, Marin-Fernandez B, Aguinaga-Ontoso I, Kramer A. Risk factors of herpes simplex type 1 (HSV-1) infection and lifestyle factors associated with HSV-1 manifestations. Eur J Epidemiol.2001; 17: 885-890. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12081109

Sign up for Article Alerts