Bioactive Vitamin D Therapy to Treat Secondary Hyperparathyroidism in Pre-Dialysis Chronic Kidney Disease: A Systematic Review?
Jason J. Bucci1, Abigail C. Staples2 and Karen E. Hansen2*
1Boston University Medical Campus, USA
2Department of Medicine, University of Wisconsin School of Medicine & Public Health, Room 4124 Medical Foundation Centennial Building, 1685 Highland Avenue, Madison, WI, 53705-2281, USA
*Address for Correspondence: Hansen KE, Department of Medicine, University of Wisconsin School of Medicine & Public Health, Room 4124 Medical Foundation Centennial Building, 1685 Highland Avenue, Madison, WI, 53705-2281, USA, Tel: 608-263-3457; Fax: 608-263-7353; E-mail: keh@medicine.wisc.edu
Submitted: 01 August 2017; Approved: 16 August 2017; Published: 19 August 2017
Citation this article: Bucci JJ, Staples AC, Hansen KE. Bioactive Vitamin D Therapy to Treat Secondary Hyperparathyroidism in Pre-Dialysis Chronic Kidney Disease: A Systematic Review. Int J Clin Endocrinol. 2017;1(1): 025-032.
Copyright: © 2017 Hansen KE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Bone mineral density; Chronic kidney disease; Falls; Secondary hyperparathyroidism; Survival, Vitamin D
Download Fulltext PDF
Background: Chronic kidney disease affects one in five adults ≥65 years old, over half of whom develop secondary hyperparathyroidism. In observational studies, secondary hyperparathyroidism is associated with low bone mineral density and excess vascular calcification, presumably leading to surplus fractures, more cardiovascular events and shortened lifespan. Several bioactive vitamin D analogs are FDA approved to treat secondary hyperparathyroidism and published algorithms direct their clinical use. However, whether such therapy improves patient outcomes is uncertain. The purpose of this review is to summarize evidence derived from clinical trials describing the benefits of treating secondary hyperparathyroidism in patients with pre-dialysis chronic kidney disease.
Methods: We performed a systematic review of the literature to identify trials using bioactive vitamin D (calcitriol, alfacalcidol, paricalcitol and doxercalciferol) to treat secondary hyperparathyroidism in chronic kidney disease patients. We focused on changes in bone mineral density, fractures, falls, vascular events and lifespan, resulting from treatment of secondary hyperparathyroidism.
Results: We found weak evidence that treating secondary hyperparathyroidism improved bone mineral density, based on two studies recruiting only 62 subjects. However, neither trial required baseline secondary hyperparathyroidism or followed published treatment algorithms. We found no randomized, placebo-controlled trials that tested the effect of treating secondary hyperparathyroidism on the risk of fractures, falls, vascular events or death.
Conclusions: Randomized, double-blind, placebo-controlled trials are needed to evaluate the potential harms and benefits of treating secondary hyperparathyroidism in pre-dialysis chronic kidney disease patients.
Abbreviations
CKD: Chronic Kidney Disease; SHPT: Secondary Hyperparathyroidism; BMD: Bone Mineral Density; FDA: Food and Drug Administration
Introduction
Chronic Kidney Disease (CKD) affects approximately 13% of adults, including roughly 1 in 5 adults over 65 years old [1]. Secondary Hyperparathyroidism (SHPT) occurs in over half of patients with stage 3 and 4 CKD [2,3] and is a risk factor for both cardiovascular events [4,5] and decreased bone mineral density (BMD) [6]. These observations suggest that treating SHPT would increase BMD and reduce vascular events.
Medicare spends an estimated 20% of its budget on care related to CKD [7], largely due to acute kidney injury, renal replacement therapy and co-morbid conditions including cardiovascular events [4,8] and fractures [9-12]. If treatment of SHPT improved musculoskeletal or vascular health, such therapy could reduce health care costs for CKD patients. Several bioactive vitamin D compounds are FDA approved to treat SHPT [13]. In 2003, the National Kidney Foundation published the Kidney Disease Outcome Quality Initiative (K/DOQI) SHPT treatment algorithms [14] which outlined specific PTH targets to achieve with vitamin D therapy, based on the degree of CKD. However, in 2017 the National Kidney Foundation recommended against routine use of vitamin D analogs to treat SHPT in CKD patients [15,16]. Instead, the 2017 guidelines suggest treatment only for patients with severe and progressive SHPT [15,16].
Since SHPT commonly affects older adults, including those with osteoporosis, we sought evidence that its treatment would improve musculoskeletal and vascular health. We performed a systematic review to summarize clinical trials data evaluating the clinical outcomes of treating SHPT in patients with pre-dialysis CKD.
Pathophysiology of secondary hyperparathyroidism
Vitamin D3 is produced in the skin when 7-dehydrocholesterol interacts with ultraviolet radiation [6], and is then hydroxylated in the liver to 25(OH)D3. Within the kidneys, 1-α-hydroxylase converts 25(OH)D3 to 1,25(OH)2D3, which promotes intestinal absorption of ingested calcium and phosphorus. Impaired renal function with reduced phosphorus excretion allows serum phosphorus to rise, triggering release of FGF-23. FGF-23 promotes renal phosphorus excretion, inhibits 1-α-hydroxylase and promotes 24,25 hydroxylase enzyme activity which together reduces 1,25(OH)2D3 production. 1,25(OH)2D3 levels also decline due to reduced renal mass and subsequently lower renal 1,25(OH)2D3 synthesis. Over time, 1,25(OH)2D3 deficiency reduces calcium absorption, leading to hypocalcemia. In response to hypocalcemia, calcium receptors in the parathyroid glands trigger increased secretion of Parathyroid Hormone (PTH) [17], upregulating osteoclastic bone resorption to liberate skeletal calcium and maintain normocalcemia. PTH also up-regulates renal expression of 1-α-hydroxylase to promote increased 1,25(OH)2D3 synthesis. Eventually, skeletal resistance to PTH occurs [18], leading to further increases in PTH in attempts to maintain bone turnover and thereby sustain normocalcemia. Thus, at the potential cost of skeletal mineral, SHPT helps CKD patients maintain normocalcemia. The pathophysiology of SHPT, reviewed in greater detail elsewhere [19], suggests that its treatment would improve skeletal health.
Methods
We searched PubMed and CINAHL Plus between January 1, 1974 and June 8, 2017 to identify randomized, placebo-controlled trials in which calcitriol, paricalcitol, alfacalcidol or doxercalciferol was used to treat SHPT in adults with CKD and any of the following clinical outcomes was measured: bone mineral density, fractures, falls, vascular events or lifespan. Key search terms were: calcitriol, paricalcitol, alfacalcidol, doxercalciferol, chronic kidney disease, secondary hyperparathyroidism, vitamin D and clinical trial. We excluded reviews and trials in children, dialysis and renal transplant patients. Studies in any language were considered. We also used references listed in review articles to supplement our own literature search.
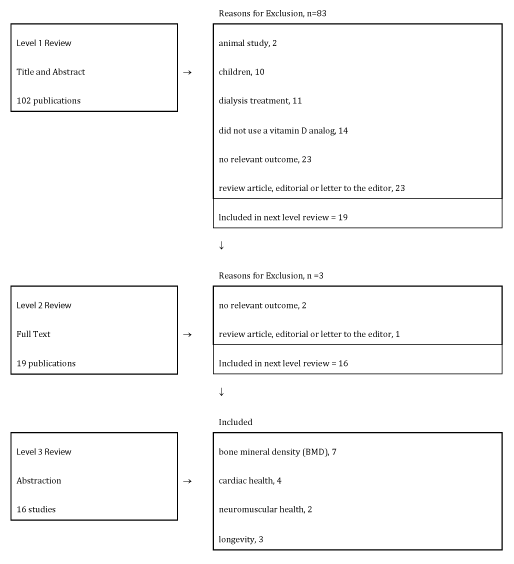
Our literature search results are summarized in figure 1. We identified 102 potentially relevant articles including 82 articles in PubMed, 7 articles in CINAHL Plus and 13 articles through reference lists. We reviewed all titles and abstracts to determine whether each study qualified for inclusion. After applying all inclusion and exclusion criteria, we identified 16 publications for inclusion in the review.
Results
Evidence that treating SHPT improves skeletal health
Low BMD is associated with increased fractures in pre-dialysis CKD patients [11, 20-24]. As a result, BMD tests can be used clinically to assess fracture risk in these patients. Indeed, in a meta-analysis [25] of 384 pre-dialysis CKD patients from three cross-sectional studies, 129 subjects experienced fractures, and their femoral neck BMD was 0.11 gm/cm2 lower than those who did not fracture (95% CI: -0.15 to -0.07, p < 0.001, I2 =30%).
In a Tufts evidence review performed for the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, 14 of 20 observational studies in 2,371 subjects with CKD found an inverse correlation between PTH and BMD [19], while the remaining studies found no relationship. However, only 2 studies recruited patients with pre-dialysis CKD. We found two additional studies; all four are summarized below and in table 1.
Using peripheral quantitative computed tomography, Tsuchida, et al. [26] found no correlation between cortical, trabecular and total ultra-distal radius BMD in 85 pre-dialysis CKD patients (Table 2). In a subset of 53 subjects returning one year later [27], the annual change in cortical, trabecular and total ultra-distal radius BMD was likewise not associated with PTH. In a cross-sectional study [28] of 113 patients with pre-dialysis CKD, subjects were divided into three groups: PTH< 60 pg/mL (normal range), PTH 60-120 pg/mL and PTH >120 pg/mL. Subjects with PTH ≥ 60 pg/mL had significantly lower spine and femoral neck, but not forearm, Z-scores than those with PTH <60 pg/mL (p < 0.05); a correlation coefficient was not reported. In a cross-sectional study of 89 patients with stage 3-4 CKD, log PTH was inversely associated with log total hip BMD (r = -0.313, p = 0.003) [3]. In 69 patients with pre-dialysis CKD, PTH was inversely correlated with 33% radius (r = -0.701, p < 0.01) but not spine BMD [29].
In summary, three of four studies in 356 patients found that hyperparathyroidism was associated with lower BMD. However, the studies did not find a preferential effect of SHPT on cortical bone. Additionally, the single prospective study [27] detected no relationship between SHPT and changes in radius BMD.
Observational data describe an inverse relationship between PTH and BMD in CKD patients, suggesting that treatment of SHPT with bioactive vitamin D might increase BMD and reduce fractures. Unfortunately, few clinical trials have tested this hypothesis. Przedlacki, et al. [30] randomized 26 subjects to one year of daily placebo. GFR was 31 (SEM, 4) mL/minute in the placebo and 22 (SEM, 3) mL/minute in the calcitriol arms, respectively. While SHPT was not an inclusion criterion, baseline PTH levels were 150 (SEM, 26) ng/L and 123 (SEM, 26) ng/L in the calcitriol and placebo arms, respectively (Table 2). Femoral neck and spine BMD increased by 3.4% and 3.9% in the calcitriol arm, but decreased by 2.2% and 0.6% in the placebo arm (p <0.01, all between-arm comparisons). In a second study, Rix, et al. [31] randomized 36 subjects with GFR between 10 to 60 mL/min to placebo or alfacalcidol for 18 months. Again, SHPT was not an inclusion criterion, but PTH levels were 183 pg/mL and 121 pg/mL in alfacalcidol and placebo arms, respectively (Table 2). Subjects randomized to alfacalcidol experienced a 4.2% gain in spine and 3.0% gain in total hip BMD, relative to placebo-treated subjects (p < 0.05, all between-arm comparisons). While both studies suggest that treating SPHT increases BMD, neither study required SHPT for study inclusion nor followed the K/DOQI treatment algorithms.
We found only one randomized trial mentioning fracture as a function of calcitriol vs. placebo therapy in pre-dialysis CKD patients [32]. However, only 7 of the 17 subjects had SHPT. During the one-year trial, one participant per arm sustained a fracture, as reported to authors of a subsequent meta-analysis [6].
A 2009 Cochrane meta-analysis [6] summarizing the efficacy of bioactive vitamin D compounds in pre-dialysis CKD criticized all three studies, citing uncertain blinding procedures, unclear randomization methods and lack of intent-to-treat analysis. Using data from 7 studies in 612 participants, the meta-analysis also concluded that bioactive vitamin D therapy for SHPT was associated with increased risk of hypercalcemia (RR 3.04, 95% CI 1.17 to 7.90; I2=0%).
In conclusion, three of four observational studies in 356 patients suggest that PTH is inversely correlated with BMD in CKD patients. Additionally, BMD predicts fracture risk in pre-dialysis CKD. However, we found limited clinical trials data confirming that treatment of SHPT with bioactive vitamin D increases BMD, and no evidence that treatment reduces fractures.
Evidence that treating SHPT improves cardiovascular health
Cardiovascular events are the primary cause of death in patients with CKD [33]. Vascular calcification and Left Ventricular Hypertrophy (LVH) are more prevalent among patients with CKD, and both conditions are independent risk factors for cardiovascular disease [34-36]. Additionally, hyperparathyroidism [4] and hypovitaminosis D [37] are independent predictors of vascular calcification. Therefore, treating SPHT might reduce vascular calcification, subsequently lowering rates of cardiovascular events and prolonging life. On the other hand, hypercalcemia or hyperphosphatemia resulting from bioactive vitamin D therapy might promote vascular calcification, thereby increasing the risk of vascular events and shortening life span.
To date, there are no randomized, placebo-controlled trials evaluating whether treatment of SHPT reduces cardiovascular events in pre-dialysis CKD patients. However, we found four studies evaluating the efficacy of vitamin D therapy on cardiac function. Singh, et al. [38] randomized 30 pre-dialysis CKD patients with GFR <30 mL/min and PTH >180pg/mL to daily calcitriol 0.5μg or placebo for 12 weeks (Table 2). Calcitriol users experienced decreased early atrial filling velocities (0.696 ± 0.089 m/s to 0.680 ± 0.084 m/s, p < 0.001), in contrast to the placebo group. There were no significant between-arm changes in left ventricular dimensions or left ventricular ejection fraction. No cardiovascular events were reported.
In another trial, Ivarsen, et al. [39] randomized 13 patients with stage 4 CKD, SHPT (PTH > 3 times upper limit of normal) and LVH to alfacalcidol 0.5μg or placebo daily for 6 months (Table 2). Fractional shortening (a two-dimensional estimate of left ventricular function) increased by 20% in the alfacalcidol group, but remained stable among placebo users (p < 0.05). Investigators found no between-arm changes in left ventricular mass, blood pressure, 25(OH)D or phosphate levels.
In a third study called Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity or “PRIMO” [40], 227 patients with stage 3-5 CKD, PTH 50-300 pg/mL and LVH were randomized to 48 weeks of placebo or paricalcitol 2 µg/d (Tables 2,3). Paricalcitol normalized PTH within one month yet had no effect on the primary outcome of left ventricular mass index, nor measures of diastolic dysfunction. Hypercalcemia occurred in almost one in four individuals randomized to paricalcitrol, but in only 1% of individuals in the placebo arm (p < 0.001, Table 3) indicating potential excess harm of paricalcitol. Interestingly, fewer in the paricalcitol arm experienced cardiovascular events subjects (1 versus 7 subjects, p = 0.03).
A final study evaluated the efficacy of oral paricalcitol on retarding cardiac hypertrophy, reducing inflammation and atherosclerosis in patients with stage 3-5 CKD. The “OPERA” study [41] randomized 60 patients with stage 3-5 CKD, PTH ≥55 pg/mL and LVH to paricalcitol 1 µg/d or placebo for one year (Tables 2, 3). Although paricalcitol reduced PTH by a median of 86 pg/mL, researchers detected no between arm differences in the primary outcome of left ventricular mass index. Even though the dose of paricalcitol was lower in OPERA than in the PRIMO trial, hypercalcemia affected almost half of subjects randomized to paricalcitol but only 3% of subjects assigned to placebo (p < 0.001). Fewer paricalcitol treated subjects had cardiovascular events requiring hospitalization (0 versus 5 subjects, p-value not reported). Interestingly, a post-hoc analysis [42] of the trial found that paricalcitol reduced left atrial enlargement index, a marker of cardiovascular events.
In summary, while hyperparathyroidism and CKD are strong predictors of cardiovascular disease, we found scant evidence that treatment of SHPT in pre-dialysis CKD patients directly reduces vascular events or mortality. The surrogate marker of left ventricular mass was not affected in four studies of vitamin D analogs versus placebo. Two recent trials, PRIMO and OPERA, highlighted the high incidence of hypercalcemia with the use of bioactive vitamin D. It is possible that lower doses of paricalcitol, or treatment of only patients with severe SHPT, would reduce the incidence of hypercalcemia. However, the risks of bioactive vitamin D prompted the KDIGO work group to recommend against routine treatment of SHPT in the 2017 KDIGO Clinical Practice Guidelines for the Diagnosis, Prevention and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder. It should be noted the work group did not achieve universal agreement against routine treatment of SHPT. The reason for lack of consensus was not explained, but might be due to the milder degree of SHPT in the PRIMO and OPERA studies, or the lower rate of cardiovascular events in subjects randomized to paricalcitol.
Evidence that treating SHPT improves neuromuscular health
Cholecalciferol or ergocalciferol is often recommended to improve muscle strength and decrease falls in older adults [43,44]. CKD is an independent risk factor for falling [12,45-48], and nearly all osteoporotic fractures occur after a fall [49]. Regrettably, few studies have addressed whether vitamin D reduces falls in pre-dialysis CKD patients with SHPT.
Dukas, et al. [47] performed a post-hoc analysis of subjects with CrCl <65 mL/min enrolled in a 36-week double-blind placebo-controlled trial to assess efficacy of alfcalcidol on falls. However, the study did not specifically recruit patients with SHPT, and basal PTH levels were largely normal (40 ± 17 and 29 ± 25 pg/mL in the alfacalcidol and placebo arms, respectively) (Table 2).
In a randomized double-blind, placebo-controlled trial that included 106 subjects with CrCl <60 mL/minute [48], Gallagher, et al. evaluated the effect of 3 years of conjugated equine estrogens and calcitriol on BMD and falls. However, subjects’ baseline serum PTH levels were 37 pg/mL, suggesting that few subjects had SHPT (Table 2).
In summary, post-hoc analyses of two trials suggest that calcitriol and alfacalcidol reduce falling in pre-dialysis CKD patients. However, we found no randomized, placebo-controlled trials that specifically recruited subjects with SHPT, or tested the effect of the K/DOQI treatment algorithms on risk of falling.
Evidence that treating SHPT prolongs life
SHPT is associated with increased mortality in hemodialysis patients [50], and bioactive vitamin D therapy is associated with improved survival in this population [51-54]. However, such therapy might simply be a marker of greater access to health care, including better management of cardiovascular risk factors. It is therefore critical to evaluate whether treating SHPT improves survival among patients with pre-dialysis CKD.
In a retrospective single-center observational study of 520 men with stage 3-5 CKD not on dialysis [54], calcitriol therapy was associated with decreased mortality (incidence rate ratio 0.35, 95% CI 0.23 to 0.54) compared with no therapy (Table 2). Shoben, et al. [53] compared mortality rates in calcitriol users and nonusers in a retrospective study of 1418 Veterans Affairs patients with stage 3-4 CKD and PTH >70 pg/mL (Table 2). The mortality risk, after adjustment for age, gender and race, was 26% lower (95% CI, 5% to 40%) among calcitriol users, compared to nonusers. In a third retrospective study [55] of 665 patients with pre-dialysis CKD, hazards of cardiovascular events, cardiovascular death and death from any cause was compared between patients treated with alfacalcidol or no therapy (Table 2). Alfacalcidol was associated with lower all-cause mortality in unadjusted models (hazard ratio 0.47, 95% CI 0.27 to 0.81) but not in multivariate models controlling for age, gender and co-morbidities (hazard ratio 0.80, 95% CI 0.44 to 1.46).
Two meta-analyses of these three retrospective studies concluded that bioactive vitamin D reduced mortality. The hazard ratio for death was 0.59 (95% CI 0.35 to 0.99, I2=79%) in one meta-analysis [56] and the risk ratio for death was 0.73 (95% CI 0.55 to 0.98, I2=0%) in the other meta-analysis [57].
In summary, retrospective studies suggest that bioactive vitamin D therapy prolongs life in pre-dialysis CKD. However, we found no prospective, randomized clinical trials to confirm these retrospective study findings.
Discussion
In conclusion, observational data suggest that SHPT is associated with low BMD and excess vascular calcification, presumably contributing to fractures, cardiovascular events and shortened lifespan. Retrospective studies suggest that bioactive vitamin D might prolong life. However, we found very few prospective, randomized, double-blind placebo-controlled clinical trials confirming that treatment of SHPT affects patient-centered health outcomes such as BMD, fractures, falls, cardiovascular events or lifespan. Furthermore, over-suppression of PTH with vitamin D analogs could promote hypercalcemia and vascular calcification, and/or reduce bone turnover leading to adynamic bone disease [58]. Well-designed placebo-controlled clinical trials with relevant clinical outcomes are needed, to clarify the benefits and harms of treating SHPT in CKD patients. Given the lack of evidence that treating SHPT improves patient-centered outcomes, the 2017 National Kidney Foundation guidelines recommend against routine treatment of all CKD patients with SHPT [15].
Acknowledgement
This review received no funding, the authors declare no conflicts of interest and ethical approval and informed consent were not applicable for this review article.
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007; 298: 2038-47. https://goo.gl/bgPesM
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007; 71: 31-8. https://goo.gl/tgD9s4
- Stavroulopoulos A, Porter CJ, Roe SD, Hosking DJ, Cassidy MJ. Relationship between vitamin D status, parathyroid hormone levels and bone mineral density in patients with chronic kidney disease stages 3 and 4. Nephrology (Carlton). 2008; 13: 63-7. https://goo.gl/Pej3AC
- Siasos G, Tousoulis D, Michalea S, Oikonomou E, Kolia C, Kioufis S, et al. Biomarkers determining cardiovascular risk in patients with kidney disease. Curr Med Chem. 2012; 19: 2555-71. https://goo.gl/jKXiVd
- Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int Urol Nephrol. 2012; 44: 541-7. https://goo.gl/Qdn48H
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009; 7; CD005633. https://goo.gl/qFSp4J
- Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015; 66: 1-305. https://goo.gl/PeB94p
- Pugliese G, Solini A, Bonora E, Orsi E, Zerbini G, Giorgino F, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis. 2011; 218: 194-9. https://goo.gl/C2LgkP
- Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007; 167: 133-9. https://goo.gl/4EFKg5
- Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006; 17: 3223-32. https://goo.gl/NZimLR
- Jamal SA, West SL, Nickolas TL. The clinical utility of FRAX to discriminate fracture status in men and women with chronic kidney disease. Osteoporos Int. 2014; 25: 71-6. https://goo.gl/USF2GK
- Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014. https://goo.gl/1kt1He
- Coyne DW, Andress DL, Amdahl MJ, Ritz E, de Zeeuw D. Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant. 2013; 28: 2260-8. https://goo.gl/EFh71K
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003; 42: 1-201. https://goo.gl/6mMGRf
- Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017; 92: 26-36. https://goo.gl/pajFhU
- Group KDIGOKC-MUW. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017; 7: 1-59. https://goo.gl/aKLy1h
- Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007; 45: 139-167. https://goo.gl/eannS7
- Bover J, Jara A, Trinidad P, Rodriguez M, Martin-Malo A, Felsenfeld AJ. The calcemic response to PTH in the rat: effect of elevated PTH levels and uremia. Kidney Int. 1994; 46: 310-7. https://goo.gl/Rfr9P8
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009: 1-130. https://goo.gl/Z4LK4X
- Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011; 22: 1560-1572. https://goo.gl/wGm6zR
- Ensrud KE, Parimi N, Fink HA, Ishani A, Taylor BC, Steffes M, et al. Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis. 2014; 63: 31-39. https://goo.gl/c69UeJ
- Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012; 7: 1130-1136. https://goo.gl/wsL1dQ
- Jamal S, Cheung AM, West S, Lok C. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int. 2012; 23: 2805-2813. https://goo.gl/yu7SCm
- Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. 2010; 21: 1371-80. https://goo.gl/3z13VW
- Bucur RC, Panjwani DD, Turner L, Rader T, West SL, Jamal SA. Low bone mineral density and fractures in stages 3-5 CKD: an updated systematic review and meta-analysis. Osteoporos Int. 2015; 26: 449-458. https://goo.gl/itrrLt
- Tsuchida T, Ishimura E, Miki T, Matsumoto N, Naka H, Jono S, et al. The clinical significance of serum osteocalcin and N-terminal propeptide of type I collagen in predialysis patients with chronic renal failure. Osteoporosis international. 2005; 16: 172-9. https://goo.gl/Z4yYFb
- Obatake N, Ishimura E, Tsuchida T, Hirowatari K, Naka H, Imanishi Y, et al. Annual change in bone mineral density in predialysis patients with chronic renal failure: significance of a decrease in serum 1,25-dihydroxy-vitamin D. J Bone Miner Metab. 2007; 25: 74-9. https://goo.gl/K3wEvW
- Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney international. 1999; 56: 1084-93. https://goo.gl/LbrBfj
- Bianchi ML, Colantonio G, Montesano A, Trevisan C, Ortolani S, Rossi R, et al. Bone mass status in different degrees of chronic renal failure. Bone. 1992; 13: 225-8. https://goo.gl/sqbu2S
- Przedlacki J, Manelius J, Huttunen K. Bone mineral density evaluated by dual-energy X-ray absorptiometry after one-year treatment with calcitriol started in the predialysis phase of chronic renal failure. Nephron. 1995; 69: 433-7. https://goo.gl/VA2cZP
- Rix M, Eskildsen P, Olgaard K. Effect of 18 months of treatment with alfacalcidol on bone in patients with mild to moderate chronic renal failure. Nephrol Dial Transplant. 2004; 19: 870-6. https://goo.gl/pNEDbx
- Baker LR, Abrams L, Roe CJ, Faugere MC, Fanti P, Subayti Y, et al. 1,25(OH)2D3 administration in moderate renal failure: a prospective double-blind trial. Kidney Int. 1989; 35: 661-9. https://goo.gl/ueDyu4
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351: 1296-305.
- Levey AS, Eknoyan G. Cardiovascular disease in chronic renal disease. Nephrol Dial Transplant. 1999; 14: 828-33. https://goo.gl/ynpD8T
- Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004; 44: 198-206. https://goo.gl/6PLLqt
- Hanada S, Ando R, Naito S, Kobayashi N, Wakabayashi M, Hata T, et al. Assessment and significance of abdominal aortic calcification in chronic kidney disease. Nephrol Dial Transplant. 2010; 25: 1888-95. https://goo.gl/kdQuUn
- Fusaro M, Tripepi G, Noale M, Vajente N, Plebani M, Zaninotto M, et al. High prevalence of vertebral fractures assessed by quantitative morphometry in hemodialysis patients, strongly associated with vascular calcifications. Calcif Tissue Int. 2013; 93: 39-47. https://goo.gl/uf89Uc
- Singh NP, Sahni V, Garg D, Nair M. Effect of pharmacological suppression of secondary hyperparathyroidism on cardiovascular hemodynamics in predialysis CKD patients: A preliminary observation. Hemodial Int. 2007; 11: 417-23. https://goo.gl/GnKnfX
- Ivarsen P, Povlsen JV, Christensen KL. Effect of alfacalcidol on cardiac function in patients with chronic kidney disease stage 4 and secondary hyperparathyroidism: a pilot study. Scand J Urol Nephrol. 2012; 46: 381-8. https://goo.gl/S22apy
- Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012; 307: 674-84. https://goo.gl/5zukjL
- Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014; 25: 175-86. https://goo.gl/gkgxwP
- Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012; 164: 902-9. https://goo.gl/x9ZBrr
- Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003; 18: 343-51. https://goo.gl/vfJR6b
- Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int. 2015; 2015: 953241. https://goo.gl/kyDQns
- Dukas L, Schacht E, Stahelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005; 16: 1683-90. https://goo.gl/Emhbde
- Dukas LC, Schacht E, Mazor Z, Stahelin HB. A new significant and independent risk factor for falls in elderly men and women: a low creatinine clearance of less than 65 ml/min. Osteoporos Int. 2005; 16: 332-8. https://goo.gl/XtfbGU
- Dukas L, Schacht E, Mazor Z, Stahelin HB. Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 ml/min. Osteoporos Int. 2005; 16: 198-203. https://goo.gl/Pppbpa
- Gallagher JC, Rapuri P, Smith L. Falls are associated with decreased renal function and insufficient calcitriol production by the kidney. J Steroid Biochem Mol Biol. 2007; 103: 610-3. https://goo.gl/PYT9vT
- Morrison A, Fan T, Sen SS, Weisenfluh L. Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon Outcomes Res. 2013; 5: 9-18. https://goo.gl/bdGhHr
- Kalantar Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006; 70: 771-80. https://goo.gl/ugn9hh
- Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005; 16: 1115-25. https://goo.gl/DaoPY4
- Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006; 70: 1858-65. https://goo.gl/Lt6zEV
- Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008; 19: 1613-9. https://goo.gl/PW4u2X
- Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008; 168: 397-403. https://goo.gl/RSJ2ok
- Sugiura S, Inaguma D, Kitagawa A, Murata M, Kamimura Y, Sendo S, et al. Administration of alfacalcidol for patients with predialysis chronic kidney disease may reduce cardiovascular disease events. Clin Exp Nephrol. 2010; 14: 43-50. https://goo.gl/sqFSej
- Zheng Z, Shi H, Jia J, Li D, Lin S. Vitamin D supplementation and mortality risk in chronic kidney disease: a meta-analysis of 20 observational studies. BMC Nephrol. 2013; 14: 199. https://goo.gl/8u17eY
- Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daures JP, Argiles A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013; 37: 239-48. https://goo.gl/fYXDQ2
- Bover J, Urena P, Brandenburg V, Goldsmith D, Ruiz C, DaSilva I, et al. Adynamic bone disease: from bone to vessels in chronic kidney disease. Semin Nephrol. 2014; 34: 626-40. https://goo.gl/DMq1ro

Sign up for Article Alerts