Practical Guide for Premium IOLs in 2017?
Gilles Lesieur*
Department of Ophthalmologist, Centre Ophtalmologique IRIDIS, Albi, France
*Address for Correspondence: Gilles Lesieur, Department of Ophthalmologist, Centre Ophtalmologique IRIDIS, 32 Place Jean Jaures, 81000 Albi, France, Tel : +33 5 63 49 47 80 ; E-mail: g.lesieur@centre-iridis.fr
Submitted: 22 March 2017; Approved: 06 April 2017; Published: 18 April 2017
Citation this article: Lesieur G. Practical Guide for Premium IOLs in 2017. Int J Ophthal Vision Res. 2017;1(1): 006-011.
Copyright: © 2017 Lesieur G. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
The use of premium IOLs requires more specifically than standard monofocal IOLs a thorough clinical and para clinical examination using modern equipments.
We will only mention micro-incision premium IOLs that are used in our daily practice. All information regarding the characteristics of all available and especially multifocal IOLs are available in the SFO 2012 Report on presbyopia [1].
Cataract surgery in 2017 must not only restore the optical media by the removal of a cloudy lens but also correct if possible all optical defects (myopia, hyperopia, astigmatism and presbyopia) without creating some with a too large corneal incision [2].
There are currently four types of premium IOLs: accommodative IOLs, IOLs with improved depth of field under development, Toric IOLs and multifocal IOLs.
We have no experience of accommodative IOLs that are widely used overseas.
The accuracy of IOLs power calculating devices using optical interferometry biometry (target +/- 0.50 D) and the evolution to astigmatism neutral micro-incisions enable us in daily practice to customize these IOLs to each individual patient with an asphericity (Z4 - 0) suited to the cornea.
This allows for partial presbyopia correction by depth of field increase. This is the first step in the development of IOLs with improved depth of field which will probably soon revolutionize our correction possibilities by IOL customization.
In this article, we will essentially focus on multifocal and toric IOLs.
For about 700,000 cataract procedures performed in 2012, we can estimate to 6.5% i.e., 45,000 toric IOLs placed in France and 4% i.e., 28,000 multifocal IOLs (25,000 multifocal IOLs and 3,000 toric multifocal IOLs).
The multifocal IOLs market progresses slowly (40% of French surgeons use multifocal versus 30% last year) and very variably according to surgical orientations, refractive or not.
It is quite different with the toric IOL market that grows very quickly with 50% of respondents using torics versus 37% last year according to Richard Gold in 2012.
Indeed 20 to 30% of cataract patients have an astigmatism superior to 1.25D and 10% of 2D or more.
This can largely vary with regions with 45% of patients having more than one diopter astigmatism according to a recent Chinese article [3].
Multifocal IOLs
Patients’ requirements have greatly increased in recent years and even perfect distance vision correction is not enough to fully satisfy a cataract surgery patient.
This is particularly notable in a myopic patient who had no problem with his intermediate and near vision before his cataract and his corrective surgery. Monocular vision and/or depth of field increase by spherical aberrations management are not always sufficient to reach the goal of less spectacle dependence.
Such patients will be preferentially attracted by multifocality if they accept the induced visual compromise (halos and reduced contrast sensitivity at low luminance).
Which patients are suitable for this surgery?
The most suitable patient is the one who strongly desires not to wear glasses after having eliminated medical contra-indications and exposed side effects especially halos.
Thus in our activity we perform more PRELEX® or PREs biopic Lens Exchange-acronym created by Kevin Waltz, et al. [4] in the early 1990s than multifocal implantation in cataract patients.
Patients with significant night activity should be avoided as halos at night may disturb patients especially when driving.
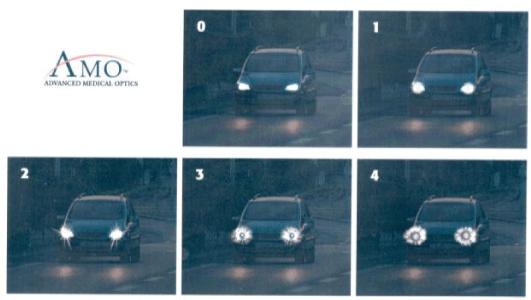
These halos disappear for 20% of patients during the first month and for 40% of patients during the first year presumably by a Neuro-adaptation phenomenon.
They persist to varying degrees for the remaining 40% without significant reduction in activities.
Clear information using a booklet with halos simulation (figure 1) or more sophisticated computer software may be used.
Which preoperative assessment?
An orthoptic assessment will be done to eliminate any microtropia. Analysis of the cornea must be scrupulous and any disease of the tear film must be treated beforehand because meibomian gland dysfunction can greatly disturb patients postoperatively.
Search of the dominant eye will be systematically determined using stenopeic hole (muscular dominant eye) in cataract cases and adding + 0.75 or more (cortical dominant eye) to the best refraction in case of clear lens exchange.
New apodized diffractive IOLs being pupil-dependent, photopic (Scheimpflug data) and mesopic (Colvard Pupillometer) measurement of the pupil will avoid narrow photopic or over dilated scotopic pupils.
Limits of 2mm in photopic and 5 mm in scotopic will avoid any pupillary refractive disorder postoperatively.
A mean 2.92 mm ± 0.55 mm pupil was found during the presentation of the Phys IOL Fine Vision Trifocal IOL at the ASCRS 2012 meeting in Chicago (free paper).
Pathological pupils are absolute contra-indications to this surgery.
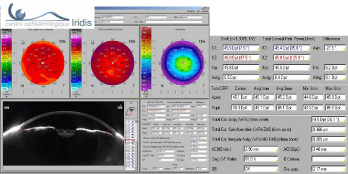
Topographic analysis in the best case using Scheimpflug camera (Pentacam type) will eliminate pathological (fruste keratoconus) and irregular corneas.
Scheimpflug camera analysis allows quantifying the corneal irregularity (Total Cor. Irregular. Astg), which will be at best lower than 0.300 m (figure 2). Multifocal implantation is possible up to 0.500 m but contra-indicated beyond [5].
This analysis is essential especially for patients who have received previous refractive surgery.
IOL calculation should be made with great care using an optical interferometry biometer to avoid errors beyond +/- 0.50 D and if possible should be made several times.
In practice for PRELEX we make two measurements on the dominated eye and three measurements on the dominant eye at two preoperative and one postoperative examination on the first eye (non dominant eye operated firstly).
The formulas used are SRK-T, Hoffer Q and Haigis.
The SRK-T formula is suitable in the great majority of cases, Hoffer Q is our preferred for hyperopic cases and Haigis for atypical eyes.
The Haigis L formula is used for patients with a history of corneal surgery (to be compared with the online calculator on the SAFIR website https://www.safir.org or ASCRS website https://iolcalc.org and with the Pentacam EKR calculator), which does not always avoid refractive errors…
The Haigis L formula is used for patients with a history of corneal surgery (to be compared with the online calculator on the SAFIR website https://www.safir.org or ASCRS website https://iolcalc.org and with the Pentacam EKR calculator), which does not always avoid refractive errors…
Astigmatism is a factor of patient dissatisfaction.
A residual astigmatism lower than 0.50D does not seem to impair visual acuity [6], but we systematically treat astigmatism with toric lens if possible with the goal of no residual astigmatism.
Corneal limbal incisions could be performing to treat lower astigmatism.
You have also to adjust astigmatism correction with aging. For example at 60 years old you can let WTR astigmatism of + 0.50D, however zero astigmatism is the best choice for an 80 years old patient.
The Scheimpflug analysis can again maximize results by analyzing the anterior and posterior surface of the cornea and the axis of the total cornea.
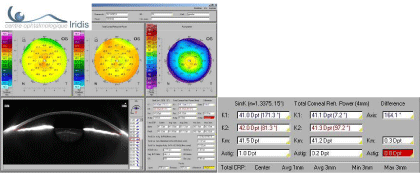
In the following case, treatment of the one diopter direct astigmatism in the anterior cornea will not be necessary because it is actually 0.2D in the total cornea (Figure 3).
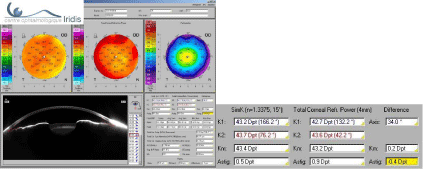
This other case confirms the benefit of analyzing the total cornea with a different axis of astigmatism between the anterior surface + 0.5 D @ 76.2° and the total cornea 0.9 D @ 42.2° (Figure 4).
Pathological capsular bags or capsular bags at risk because of uncontrolled healing should be avoided in order to prevent any decentration of these IOLs.
Finally a macular OCT analysis is performed when there is a doubt at fund us examination in order to eliminate an incipient macular traction syndrome or Epiretinal Membrane (ERM) [7].
This does not prevent the occurrence of ERM after postoperative PVD but it seems essential to us from a clinical and forensic perspective in order to anticipate a cystoid macular edema (Cumulative Cystoid Macular Edema rates after large series of MICS IOL implantations, ASCRS 2014 Boston, free paper).
Which IOL types should be used?
The history of multifocal is made of hope and disillusion; this explains the low development of this type of implantation.
In the 80s, the 3M Company has developed the first diffractive IOL with imperfect results because of incision size (7 mm in extra capsular) and IOL calculation by a less accurate US biometry.
The development of refractive IOLs in the 90s helped develop the concept at a time when surgery was revolutionized by phacoemulsification but with a lack of near correction.
This has led some surgeons to develop as Jacobi in 1999 diffractive optics with far predominance for the dominant eye and near predominance for the dominated eye. The mix and match concept was born to compensate the inability of one single IOL to provide patients with perfect far and near correction.
We have used the concept at this time with the AMO SA40N refractive IOL on the dominant eye and IOL tech MF4 on the dominated eye (SAFIR 2001 free paper).
The development of new bifocal diffractive implants in the 2000s (Alcon ReStor and Carl Zeiss Meditec AT Lisa) has enabled a further boost but with a lack of intermediate vision correction.
The last decisive change occurred in 2011 with the trifocal IOL (Phys IOL Fine Vision) that restores the three distances of far intermediate and near vision.
Mix and match are no longer required in 2014 with the use of trifocal diffractive IOLs in both eyes [8-9-10], which largely facilitates IOL calculation.
Which surgical technique?
It seems to us that sub 2mm micro incision (CMICS or BMICS) should be the rule in order to prevent any astigmatism induced by the incision and the risk to increase high order aberrations [2].
If topical anesthesia is becoming increasingly popular, we have chosen since 2012 to systematically perform sub-Tenon anesthesia to avoid any discomfort during the procedure [11].
The second eye is operated on 2 to 5 days after the first one to eliminate any trouble due to an eventual anisometropia.
Which postoperative follow up?
An examination is performed at one month systematically with treatment of any eventual residual blepharitis which may largely disturb patient’s vision.
In case of resisting blepharitis, we use heating glasses (Blephasteam https://www.blephasteam.fr) that can treat the symptoms and reassure patients who see their vision improve immediately after a single session.
Minimal refractive errors may be treated from the third month (bag healing) using Lasik (hypermetropia) or PRK (myopia) and earlier in case of major error in IOL calculation by IOL replacement.
Toric IOLs
Uncorrected astigmatism creates blurred vision, the need for glasses, which results in optical aberrations especially for progressive lenses, and a reduction of the visual field in particular for astigmatisms superior to 2D [12].
Indeed some patients do not tolerate this type of correction using glasses.
Which patients are suitable for this surgery?
All patients with astigmatism can benefit from this type of IOL.
Indeed very low astigmatisms may be adjusted by relaxing incisions using diamond knife with good accuracy.
Which preoperative assessment?
The same clinical and para clinical assessment as for multifocal IOLs is performed to define the toric IOL selection.
Examinations should be repeated when there is a discrepancy between different devices with regards to astigmatism axis or power. We must remember the importance of the tear film and its prior treatment if necessary.
Corneas with too much irregular astigmatism are not good indications (re: Total Cor. Irregular. Astg on Pentacam).
In contrast, asymmetric but regular astigmatism gives excellent results.
This is of major interest in keratoconus or post refractive surgery when calculation with online calculator is sometimes difficult for these non-standard IOLs.
The axis of total astigmatism from Scheimpflug topographers should be considered rather than anterior astigmatism alone (Figure 4).
Many works under the leadership of Douglas D. Koch, et al. [13] now stress the importance of calculating astigmatism of the posterior surface of the cornea. It seems desirable to take it into account, although the impact seems low, around 0.50D under-correction for indirect astigmatism (current study).
Current calculations that are solely based on anterior corneal astigmatism result in over-correction for with-the-rule or direct astigmatism and under-correction with against-the-rule or indirect astigmatism.
Finally, the patient’s age should be considered since astigmatism (normal + 0.75D @ 90°) reverses with aging of the eye and a young patient should be treated differently from an elderly one (Figure 5).
Which IOL type and which calculator should be used?
The prerequisite is an IOL as stable as possible in the capsular bag. The axis rotation has been evaluated with different IOL models from 2.5 ± 2.6 degrees to 4.42 ± 4.31 degrees.
Vision quality will be improved especially for large pupils and high sphero-cylindrical abnormalities by the use of bitoric IOLs.
Finally, the calculator must be as complete as possible with the integration in the calculation of the anterior chamber depth to avoid correction errors for too long or too short eyes.
Use of a fixed ratio can lead to cylindrical over-correction for hyperopic eyes and under-correction for myopic eyes.
Meridian analysis, where IOL power is calculated first for the steepest meridian then for the flattest meridian allowing to predict ELP (Effective Lens Position) precisely [14], should be preferred.
These calculators are available from AMO, Rayner and Zeiss.
Final target
MULTIFOCAL 0 astigmatism or + 0.25D with the rule for young patient
MONOFOCAL Patient <65 years + 0.25D to + 0.50D with the rule (reversion with age)
Patient >65 years + 0.25D with the rule to 0D near 80 years
Who to treat?
All astigmatism
How?
With toric lens according to the calculator or with AK for very low astigmatism
Result
With the rule astigmatism treatment easier with AK or TORIC IOL and need to under correct
Against the rule astigmatism treatment more difficult with AK or TORIC and need to overcorrect by 0.50D or check posterior astigmatism.
Which surgical technique?
Sub 2mm micro incision (CMICS or BMICS) should be preferred.
Marking in lying position should be avoided as cyclotorsion induces an average error of 3°.
Horizontal axis marking performed manually is very imprecise whatever the technique (slit-lamp marking in sitting position or using a marker with level) as attested by the multitude of available instruments (videotape available on YouTube.com https://www.youtube.com/watch?v=LWCqffd7nkM).
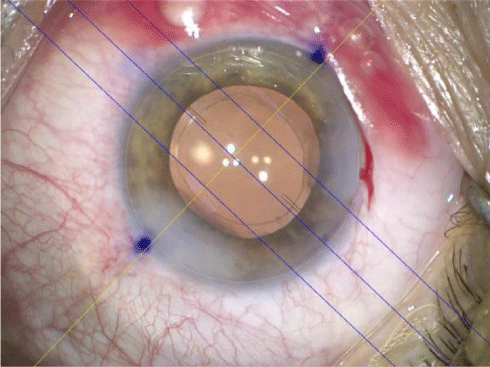
The thickness of the marking pen itself can cause an error of 5°, which means 15% loss of toric correction power (Figure 6).
Non-marking systems with image projection into the microscope should solve these technical problems (Verion Alcon, Callisto Zeiss Meditec and SG3000 SMI etc).
We are carrying out a study to compare the results of manual and automated methods using the Zeiss non-marking system.
Which postoperative follow up?
The post-operative follow is like any conventional cataract surgery and IOL axis should be checked under pupil dilation in case of bad refractive outcome.
In case of axis error or postoperative IOL rotation, IOL repositioning under topical anesthesia causes no particular problem.
Conclusion
Automation, projection of images into the microscope eyepieces and FLACS surgery (Femto Laser Assisted Cataract Surgery) are only the beginning of the development of these IOLs.
However there is a double challenge, of public health and economic, because if the interest for patients is no longer discussed it is not the same for health economics: co-payment, or the difference to be paid by the patient between a monofocal IOL (included in the GHS) and the premium IOL, is currently tolerated by health authorities in France but discussions are held since the beginning of this year to cancel it.
The possibility of inclusion on the List of Reimbursable Products and Services (LPPR) is not excluded.
If this new authority price list is frozen as are the rest of our services we can fear for the future that companies in France will reduce their investment in innovation for this added value IOLs…
- Beatrice Cochener, Catherine Albou-Ganem, Gilles renard. SFO 2012 Report: Presbyopia
- Elkady B, Alio JL, Ortiz D, Montalban R. Corneal aberrations after microincision cataract surgery. J Cataract Refract Surg. 2008; 34: 40-45. https://goo.gl/pOFbji
- Guan Z, Yuan F, Yuan YZ, Niu WR. Analysis of corneal astigmatism in cataract surgery candidates at a teaching hospital in Shanghai, China. J Cataract Refract Surg. 2012; 38:1970-1977. https://goo.gl/crwEvA
- Bruce WR, Steven D, Richard LL, Kevin W. Prelex Story chapter 190 In Mastering Refractive IOLs: The Art and Science Edited by David F. Chang. https://goo.gl/THnQRm
- Naoyuki Maeda. Assessment of corneal optical quality for Premium IOLs with pentacam. Highlights of Ophthalmology. 2011; 39: 2-5. https://goo.gl/ORdS1E
- Villegas EA, Alcon E, Artal P. Minimum amount of astigmatism that should be corrected. J Cataract Refract Surg. 2014; 40:13-19. https://goo.gl/gwTlwr
- Braga MR, Chang D, Dewey S, Foster G, Henderson BA, Hill W, et al. Multifocal intraocular lenses : Relative indications and contraindications for implantation. J Cataract Refract Surg. 2014; 40: 313-322. https://goo.gl/LQ1U5L
- Cochener B, Lafuma A, Khoshnood B, Courouve L, Berdeaux G. Comparison of outcomes with multifocal intraocular lenses: a meta analysis. Clin Ophthalmol. 2011; 5: 45-56. https://goo.gl/O1J5LV
- Cochener B, Vryghem J, Rozot P, Lesieur G, Heireman S, Blanckaert JA, et al. Visual and refractive outcomes after implantation of a fully diffractive trifocal lens. Clin Ophthalmol. 2012; 6: 1421-1427. https://goo.gl/Uf2xSf
- Lesieur G. Outcomes after implantation of a trifocal diffractive IOL. J Fr Ophtalmol. 2012; 35: 338-342. https://goo.gl/1k8TV5
- Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: Guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J Cataract Refract Surg. 2012; 38: 1086-1093. https://goo.gl/gzmlt1
- Visser N1, Bauer NJ, Nuijts RM. Toric Intraocular lenses: Historical overview, patient selection, IOL calculation, surgical techniques, clinica loutcomes, and complications. J Cataract Refract Surg 2013 ; 39: 624-637. https://goo.gl/Sj3HDL
- Koch DD1, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012; 38: 2080-2087. https://goo.gl/Bprvls
- Fam HB, Lim KL. Meridional analysis for calculating the expected sphero cylindrical refraction in eyes with toric intraocular lenses. J Cataract Refract Surg. 2007; 33: 2072-2076. https://goo.gl/1ufKax


Sign up for Article Alerts