Activity of the hydroethanolic extract of Buddleja brasiliensis Jacq. ex Spreng. Fam. on acute pain induced in mice?
Carlos CJ. Almanca1 and Bruno G. Marinho2*
1Department of Veterinary Medicine, Federal University of Espirito Santo, Alegre, ES, Brazil
2Laboratory of Pharmacology, Department of Physiological Sciences, Federal Rural University of Rio de Janeiro, Seropédica, RJ, Brazil
*Address for Correspondence: Bruno G. Marinho, Department of Physiological Sciences, Laboratory of Pharmacology, Federal Rural University of Rio de Janeiro, BR465, Km07, 23890-000, Seropédica, RJ, Brazil, Tel: +55212682; E-mail: brunomarinho@ufrrj.br; bruno.marinho78@hotmail.com
Submitted: 19 June 2017; Approved: 02 July 2017; Published: 05 July 2017
Citation this article: Almanca CCJ, Marinho BG. Activity of the Hydroethanolic Extract of Buddleja brasiliensis Jacq. Ex Spreng. Fam an Acute Pain Induced in Mice. Int J Pain Relief. 2017;1(1): 001-007.
Copyright: © 2017 Marinho BG, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Buddleja brasiliensis; Antinociception; Mice; Medicinal plants
Download Fulltext PDF
Buddleja brasiliensis is a species found in Southern Brazil, being popularly used in the treatment of inflammatory, respiratory and rheumatic diseases. From the genus Buddleja, several types of chemical compounds have been isolated, including flavonoids and other shikimate-derived compounds. The aim of this study was to study the antinociceptive activity of the hydroethanolic extract of Buddleja brasiliensis in acute pain models induced in mice. Male Swiss mice (20-22 g) were used in models of acute pain (Acetic acid-induced abdominal writhing, formalin, tail flick and hot plate tests) and in model for evaluation of spontaneous motor performance (open field test). Furthermore, we evaluate the possible action mechanism of B. brasiliensis using naloxone, L-NAME, glibenclamide, atropine, mecamylamine and ondansetron in tail flick and hot plate tests. The hydroethanolic extract from the B. brasiliensis was administered orally at doses of 50, 100 and 300 mg/ kg. The chemical analyzes performed in the phytochemical screening revealed the presence of flavonoids, triterpenes, phenylethanoid glycosides and phenolic acids. The extract showed antinociception properties in models of acute pain induced by chemical and thermal stimuli and the prior administration of naloxone reduced the antinociceptive effect of B. brasiliensis. The results showed that the hydroethanolic extract of B. brasiliensis produced central antinociceptive effect, suggesting the participation of the opioid system. The extract did not develop symptoms of toxicity.
Introduction
The pain correspond a subjective response that the organism presents when it is submitted to some potentially harmful stimulus. It is a protective function, representing in many cases, the only symptom for the diagnosis of various diseases, but when it is persistent, it provokes negative emotional reactions, becoming debilitating and causing suffering, and causing damage in social and economic relations. Pain should therefore be rapidly and effectively controlled and treated [1].
Pharmacological control of pain basically involves two strategies. The first is the use of drugs that directly block the mechanism of conduction of pain, resulting in antinociception; these drugs are represented by opioids, such as morphine, codeine, fentanyl and tramadol, which directly abolish nociceptive transmission by binding to opioid receptors [2]. The second strategy involves the use of drugs that inhibit the sensitization of nociceptors and, therefore, the development of hypernociception; this is the main mechanism of action of drugs that inhibit the cyclooxygenase, non-steroidal anti-inflammatory drugs (NSAIDs) [3].
Phytotherapy is the basis of modern pharmacology; it is also the oldest medical system in the world. The World Health Organization (WHO) defines a medicinal plant as "any plant species, cultivated or not, used for therapeutic purposes" [4]. Medicinal plants are important for pharmacological research and drug development, not only when their constituents are used directly as therapeutic agents but also as materials for synthesis, or models for pharmacologically active compounds [5].
The genus Buddleja (Scrophulariaceae) consists of about 100 species distributed in the tropics of America, Asia and Africa [6]. Buddleja species have been used in traditional medicine. Buddleja brasiliensis is a species found in Southern Brazil, being popularly used in the treatment of inflammatory, respiratory and rheumatic diseases. From the genus Buddleja, several types of chemical compounds have been isolated, including flavonoids and other shikimate-derived compounds, such as the phenylethanoid glycosides typified by verbascoside [7]. The aim of this study was to study the antinociceptive activity of the hydroethanolic extract of Buddleja brasiliensis in order to prove its use by the native population and to establish a new alternative in the treatment of algic conditions.
Materials and Methods
Animals
Male Swiss mice (20 – 22 g) were obtained from our animal facility. The animals were maintained in a room with a controlled temperature (22 ± 2 °C) and a 12 h light/dark cycle with free access to food and water. Eight hours before each experiment only water was available to animals, to avoid interference of food with absorption of the drug. The experimental protocol was approved by the Ethics Committee on the use of animals of Institute of Biological Sciences and Health of the Federal Rural University of Rio de Janeiro (CEUA-IB – UFRRJ) under number 008/2015.
Plant material
The leaves of Buddleja brasiliensis, Scrophulariaceae, were collected in the city of Muniz Freire, ES, Brazil (20º 26’ 19’’ S e 41º 23’ 44’’ W) in March 2009. The plant was authenticated by Érika Von Sohsten de Souza Medeiros (Department of Systematic Botany, Botanical Garden of Rio de Janeiro, Brazil) and a voucher specimen was deposited in the Herbarium of the Botanical Garden under the number RB492873.
Preparation of the plant extract: The leaves (250 g) were grounded and extracted with 70% hydroethanolic solution for 72 h, then filtered and exposed again to extraction with hydroethanolic solution. To obtain the crude hydroethanolic extract the filtrates were concentrated in a rotary vacuum evaporator. The yield of the extract was about 25% (w/w). The material was stored at -20°C until use.
Phytochemical analysis: The hydroethanolic extract was fractionated by solvent partition dissolving it in ethanol/water (7:3) and the solution was submitted to successive extractions with hexane (n - C6H14), dichloromethane (CH2Cl2), ethyl acetate (EtOAc) and butanol (BuOH) yielding, after distillation of the solvents. The identification of the chemical constituents was performed through spectra analysis by infrared and nuclear magnetic resonance 1H and 13C spectroscopy using uni and bi-dimensional techniques, and compared results with the literature.
Chemicals: The following substances were used: acetic acid (Vetec, Rio de Janeiro, Brazil), formalin (Merck, Darmstadt, Germany), glibenclamide, ondansetron, L-NAME, mecamylamine, atropine and dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO, USA), morphine and naloxone (Cristália, São Paulo, Brazil).
Treatments
Increasing doses of the B. brasiliensis were administered orally (50, 100 and 300 mg/ kg – p.o.). Morphine was used as positive controls. The dose of morphine (5.01 [2.47 – 8.68] mg/ kg) and (8.15 [6.24 – 10.44] – p.o. - opioid analgesic drug) were obtained by calculating the ED50 (confidence limits) in acetic acid-induced abdominal writhing test and Tail Flick test, respectively, that were performed beforehand. The ED50 value for the antinociceptive action was obtained by fitting the data points representing the antinociceptive effect demonstrated in this model by nonlinear regression using Graph Pad Prism software version 6.0 (San Diego, California, USA) (data not shown).
Different groups were treated orally with saline solution, vehicle, morphine or the B. brasiliensis (50, 100 and 300 mg/ kg) for evaluation in the Acetic acid-induced abdominal writhing, formalin, tail- flick, hot-plate and open field tests. Distilled water mixed with dimethyl sulfoxide, a solubilising agent, was used as a vehicle (5%). In the control group the animals received saline solution
To evaluate the participation of specific systems on the effect shown by B. brasiliensis; Naloxone (5 mg/ kg), Nω-Nitro-L-arginine methyl ester hydrochloride (3 mg/ kg), Ondansetron (0.5 mg/ kg), Glibenclamide (1 mg/ kg), Atropine (5 mg/ kg) and Mecamylamine (1 mg/ kg) were administered intraperitoneally (i.p.) 15 min before the oral administration of 300 mg/ kg B. brasiliensis in the tail-flick and hot-plate tests.
Acetic acid-induced abdominal writhing test: Model used to screen of the antinociceptive activity [8]. It was performed by the intra peritoneal administration of acetic acid (1.2%). The count of the total number of writhes was started immediately after the acetic acid injection and remained for 30 minutes. The pattern for counting of abdominal contortions was the appearance of strong abdominal contractions, followed by trunk twisting and extension of the hind limbs and contact of the abdomen with the floor of the recipient.
Formalin test: This model was performed to discriminate between activity on inflammatory or Non-inflammatory pain. Formalin-induced behaviour was accomplished as previously described by [9]. In this test, 0.02 ml of formalin solution (2.5% v/v) was administered into the hind paw of the mice. Subsequently, the time in seconds that the mice remained licking the paw was evaluated. The evaluation was done in 2 phases: the first (neurogenic phase) between 0-5 min after the formalin injection and the second (inflammatory phase) between 15-30min after the injection.
Tail flick test: This model was used to evaluate spinal antinociceptive activity, as previously described by [10]. The mice were placed in an acrylic tube prior to each determination and then placed on equipment to perform the tail-flick test. A light beam was focused approximately 4 cm from the tip of the tail and the tail withdrawal latency was recorded automatically. The light intensity was adjusted for baseline values between 3 - 5 s; this intensity was not changed and the animals that had baseline values outside these limits were excluded from the experiment. Measures of latency time (LT) were made at intervals of 20 min. The first two measures were made before drug administration. The average of these measures was called the ‘baseline’. In order to evaluate the contribution of different endogenous systems on the antinociceptive activity of the extract experimental groups that received specific antagonists prior to administration of the extract were used. After determination of baseline latency, mice received the extract, and the reaction latency was determined in different times after injection. The tail-flick latencies were converted to the percentage of increase over the baseline (IBL) according to the following formula:
Hot plate test: The procedure used was as described previously [11]. This model was used to evaluate supra-spinal antinociceptive activity. The animals were placed on a hot plate set at 55 ± 1°C. The reaction time (RT) was recorded when the animals licked their hind paws or jumped. Two measures were taken before the administration of substances and six measures were taken after the application of substances at 30-min intervals. The mean of RTs obtained before the administration of the substances is called the baseline. In order to evaluate the contribution of different endogenous systems on the antinociceptive activity of the extract experimental groups that received specific antagonists prior to administration of the extract were used. The mean RT obtained before administration of substances was considered the baseline (BL). Antinociception was quantified as the percentage increase over the baseline shown in each measurement time, calculated using the following formula:
Open-field test: This model was realized to measure the level of spontaneous locomotor activity in mice and was performed according to the method described by [12]. Five days before behavioural testing, each animal was handled daily for a few minutes. The mice were placed individually in the center of the observation chamber (60 minutes after oral administration of substances), in which the floor was divided into 50 squares (5 x 5 cm). The mice explored freely the observation chamber for 5 min. The result is based on the total number of squares covered by the animals.
In vivo toxicological evaluation
An acute toxicity test was performed according to the WHO guidelines [13]. Acute toxicity was determined following the experimental model described previously by [14]. A single oral dose of B. brasiliensis (1000 mg/ kg) was administered to a group of ten mice. The animals were placed in an observation chamber and videotaped for a period of 8 hours per day. Behavioural parameters such as: convulsion, hyperactivity, grooming, loss of righting reflex, increased or decreased respiration, and sedation were observed for a period of 7 days. A control group of 10 animals administered with the vehicle was also subjected to the same protocol.
Statistical analysis
All experimental groups consisted of 7–10 animals. The results are presented as the mean ± Standard Error of the Mean (SEM). Statistical significance between the groups was determined using one-way analysis of variance (ANOVA) followed by Bonferroni’s test for the acetic acid-induced abdominal writhing, formalin and open field tests. The statistical significance between the groups was determined by two-way analysis of variance (ANOVA) followed by Bonferroni’s test for the tail-flick and hot-plate tests. P values of less than 0.05, 0.01 and 0.001 were considered to be statistically significant.
Results
Phytochemical analysis
The results of the chemical analyzes performed in the phytochemical screening revealed the presence of flavonoids, triterpenes, phenylethanoid glycosides and phenolic acids in the hydroethanolic leaf extract of B. brasiliensis.
Acetic acid-induced writhing tests
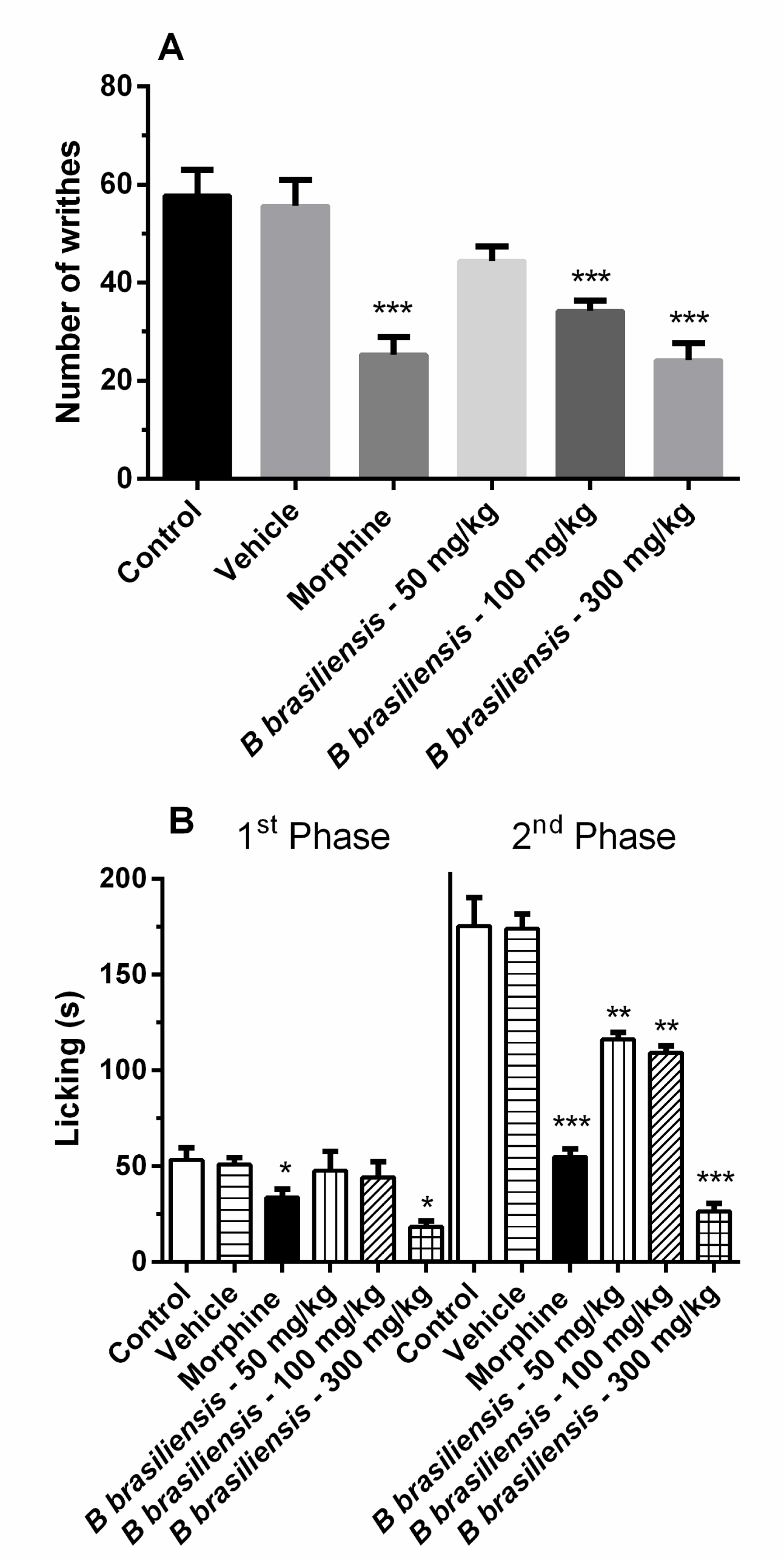
Oral administration of B. brasiliensis inhibited writhing by approximately 41% (34.2 + 2.2 writhes) and 58% (24.2 + 3.4 writhes) at doses of 100 and 300 mg/ kg, respectively. The control group showed 57.8 + 5.2 writhes, [F (5,30) = 14.11; p < 0.001]. The Morphine (5.01 mg/ kg) inhibited the number of writhes by approximately 50% compared with the control group (Figure 1A).
Formalin test
Pre-treatment with the B. brasiliensis significantly reduced the time that the mice spent licking their injected paws after formalin injection. In the first phase, the inhibitory effect was observed only with the highest dose (300 mg/ kg), while in the second phase was observed at all doses (Figure 1B).
In the first phase, the control group showed 53.2 + 6.3s (seconds), and the B. brasiliensis showed 65% (18.4 + 3.0s) inhibition at dose of 300 mg/ kg. In the second phase, doses of 50, 100 and 300 mg/ kg presented 33% (116.0 + 3.9s), 38% (109.2 + 3.6s) and 85% (26.4 + 4.0s) inhibition, respectively (Figure 1B), and control group showed (175.2 + 15.0s), [F(11,60) = 59.91; p < 0.001]. Morphine (5.01 mg/ kg) inhibited the number of licks by approximately 50% compared with the control group in both the phases.
Tail-flick test
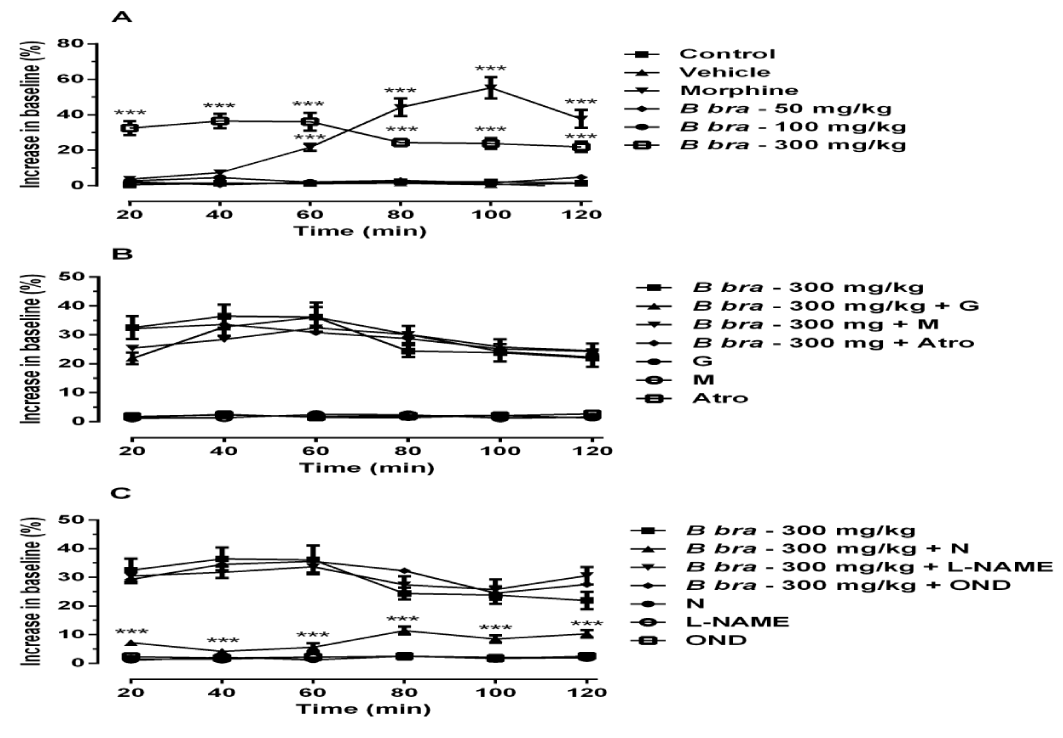
The administration of B. brasiliensis at dose of 300 mg/ kg increased significantly the latency time from the measurement time of 20 min to the measurement time of 120 min, while the morphine increased significantly the latency time from the measurement time of 60 min to the measurement time of 120 min, when compared to control group (Figure 2A), [F (5,180) = 7.81; p < 0.001].
The figures 2B and 2C show the results of administration of B. brasiliensis in association with increasing doses of Naloxone, Atropine, Mecamylamine, Glibenclamide, L-NAME and ondansetron to determine the lowest dose capable of producing a change in the antinociceptive activity of the compound.
Only naloxone reduced the antinociceptive effect of B. brasiliensis in 74%; while prior administration of mecamylamine, atropine, ondansetron, glibenclamide and L-NAME were not able to reduce the effect of B. Brasiliensis. Similar results to those obtained using the control group were observed when the antagonists were administered alone (Figure 2B and 2C).
Hot-Plate test
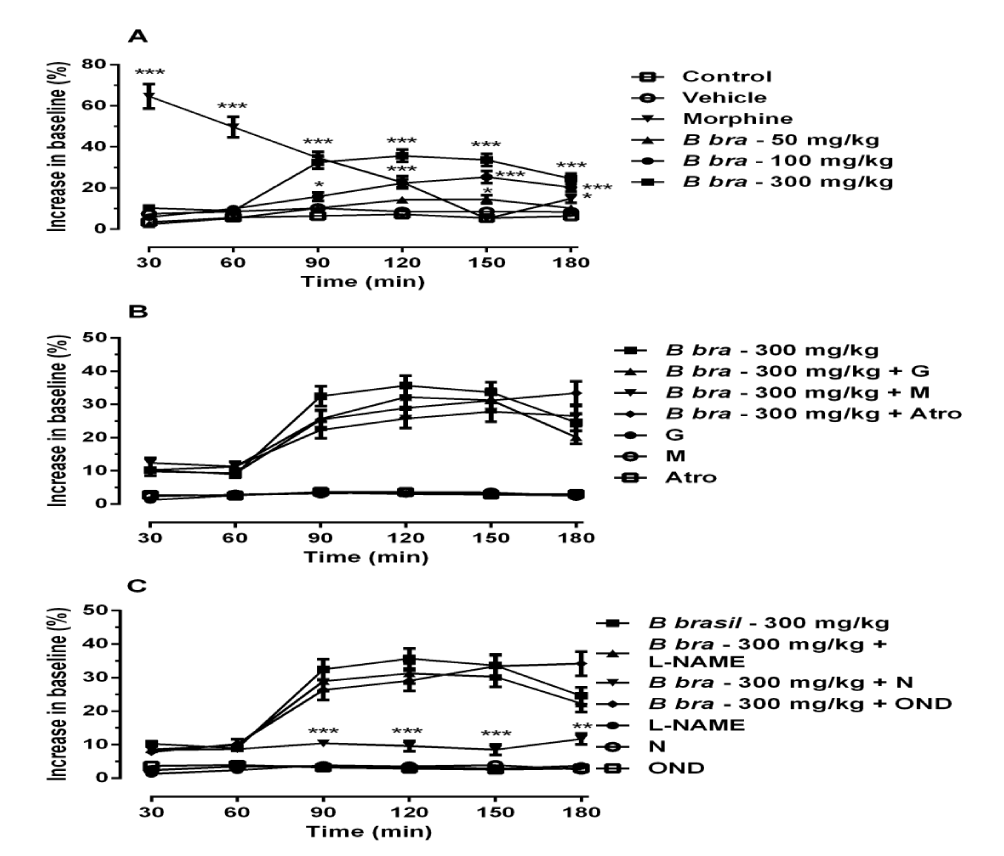
The administration of B. brasiliensis at doses of 50, 100 and 300 mg/ kg increased significantly the latency time from the measurement time of 150 min to the measurement time of 180 min, from the measurement time of 90 min to the measurement time of 180 min and from the measurement time of 90 min to the measurement time of 180 min, respectively, while the morphine increased significantly the latency time from the measurement time of 30 min to the measurement time of 120 min, when compared to control group (Figure 3A), [F(5,180) = 4.68; p < 0.001].
Figures 3B and 3C show the results of administration of B. brasiliensis in association with increasing doses of Naloxone, Atropine, Mecamylamine, Glibenclamide, L-NAME and ondansetron, and again only the naloxone reduced the antinociceptive effect of B. brasiliensis in 63%. Similar results to those obtained using the control group were observed when the antagonists were administered alone (Figure 3B and 3C).
Open Field test
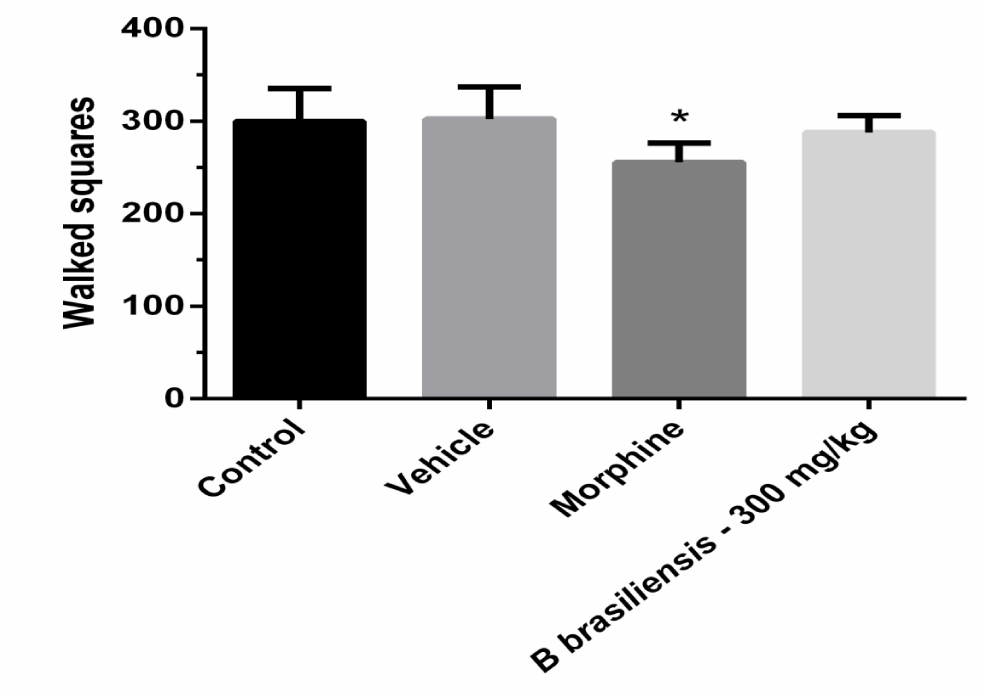
In the open-field test, the B. brasiliensis had not significant effect on locomotor activity compared with the control group at the dose of 300 mg/ kg or another dose tested (data not shown), while the morphine significantly decreased locomotor activity (Figure 4), [F(3,20) = 0.58; p < 0.05].
In vivo toxicological evaluation
The B. brasiliensis was evaluated for acute toxicity in mice. No intoxication symptoms (convulsion, hyperactivity, grooming, loss of righting reflex, respiratory rate change and sedation) were observed in the animals. The B. brasiliensis was not toxic after oral administration (1000 mg/ kg; LD50 > 1000 mg/ kg).
Discussion
The results of the present study demonstrated, that oral administration of the hydro alcoholic extract of Buddleja brasiliensis showed a potent dose-dependent inhibition of the nociceptive behavioral response in animal models of pain without causing sedation or motor impairment. This antinociceptive effect may be mediated, at least in part, by the activation of opioid system.
The intraperitoneal injection of acetic acid is a test used to search new analgesic drugs that act in peripheral, spinal and supraspinal levels. The nociceptive response of this test is produced by release cytokines such as TNF-α and IL-1β from macrophages and mast cells present in the abdominal cavity, and also involves the release and biosynthesis of prostaglandins [15,16]. The B. brasiliensis was able to reduce the abdominal writhing number induced by acetic acid with higher doses.
The formalin test is a classic acute pain animal model for study of drugs with analgesic and anti-inflammatory effects [17]. Injection of formalin into the hind paw induces a biphasic pain response; the first phase is neurogenic (early phase) and results from direct activation of primary afferent sensory neurons, predominantly by C-fiber activation, while the second phase is inflammatory (late phase) and appears to be dependent on the combination of an inflammatory reaction in the peripheral tissue and central sensitization in the dorsal horn of the spinal cord [17]. Thus, the early phase can be suppressed by the administration of centrally acting analgesics, such as morphine, while the late phase responds to various drugs with established clinical analgesic, such steroidal and non-steroidal anti-inflammatory [17]. The effect observed with the B. brasiliensis suggested that the antinociceptive action would be related to the central mechanisms. Similarly, morphine, presented the same response profile, inhibiting the two phases.
Nociceptive information is processed and integrated peripherally with the spinal and supraspinal levels to the central nervous system. Tail-flick test is a spinally integrated nociceptive reflex, while hot-plate test is a complex response which is supraspinally integrated. The B. brasiliensis showed effect in both models, confirming its central antinociceptive activity shown in the formalin test [18].
It has been shown that both cholinergic receptor agonists and acetyl cholinesterase inhibitors produce analgesia after spinal administration in rats and humans [19]. The importance of the cholinergic system in the modulation of pain is illustrated by the fact of ACh spinal release is increased as a consequence of the noradrenergic bulbospinal descending inhibition in response to noxious stimuli [20]. It has been shown that high frequency transcutaneous electric nerve stimulation in rats can inhibit hyperalgesia and this effect is dependent of cholinergic mechanisms [21].
The nitrergic pathway has an important role in the modulation of nociception [22]. The involvement of NO in nociception is dual; studies show that administration of NO donors inhibits PGE2-induced hypernociception and that this effect is reversed with the use of L-NAME, a nitric oxide synthase inhibitor, responsible for NO production [23]. However, other studies have shown the participation of the nitrergic pathway in the analgesic effect of opioids [24].
Modulation of nociception by 5-HT at the spinal level is a complex process, because it may inhibit and/or facilitate nociceptive transmission depending of the type of nociceptive stimuli and, most importantly, the nature of the 5-HT receptors expressed centrally [25]. There is evidence that the intrathecal administration of exogenous 5-HT reverses tactile allodynia in spinal nerve ligated in rats [26]. The antiallodynic effect of 5-HT has been attributed to the activation of 5-HT1/5 receptors [27], while 5-HT2/7 receptors have been associated with pro-nociception [28].
Based on this, the previous administration of Nω-Nitro-L-arginine methyl ester hydrochloride (non-selective nitric oxide synthase inhibitor), Atropine (non-selective muscarinic antagonist), mecamylamine (non-selective nicotinic antagonist), Ondansetron (5-HT3 serotoninergic antagonist), and Glibenclamide (selective ATP-sensitive K+ channel blocker) did not change the analgesic effect of B. brasiliensis. Depending of the dose and route of administration used, these antagonists could induce alterations in the nociceptive response when used alone [29-31], but at the doses used in this paper, it was not observed.
The opioid hypothesis was investigated by attempting to reverse analgesia, through prior administration of naloxone (opioid antagonist). In this regard, our results show that preadministration of naloxone was able to reverse the antinociception of B. brasiliensis, clearly demonstrating an important involvement of the extract in the opioid system activation to produce analgesia.
The open field test was used to exclude the possibility that the antinociceptive action of extract could be related to nonspecific disturbances in the locomotor activity of the animals. We observed that with effective antinociceptive dose, the hydroethanolic extract of B. brasiliensis did not alter the spontaneous motor performance of mice.
In this study four experimental models of acute pain were used to assess the analgesic property of the hydroethanolic extract of Buddleja brasiliensis leaves. This study has demonstrated the antinociceptive activity of B. brasiliensis in the models of chemical nociception induced by acetic acid and formalin, as well as in models of nociception induced by thermal stimuli (tail flick and hot plate tests). The tail-flick and hot-plate tests revealed central activity, while the formalin test investigated both central and peripheral effects. Our result suggests the antinociceptive effect of B. brasiliensis at the spinal and supraspinal levels using tail-flick and hot-plate tests. According to these results, we can infer that the antinociceptive effect of B. brasiliensis seemed to be mediated by activation of the opioid system. The absence of adverse effects and intoxication was observed in our study by the lack of symptoms of intoxication in the animals that received the B. brasiliensis orally. More studies are needed for a better understanding of its mechanism.
Acknowledgment
Thanks to the authors and to FAPERJ (Fundação de amparo à pesquisa do estado do Rio de Janeiro), CNPQ (Conselho nacional de desnvolvimento científico e tecnológico) and CAPES (Coordenação de aperfeiçoamento de pessoal de nível superior).
- Chen T, Yuan S, Yu X, Jiao L, Hu W, Chen W, et al. Effect of toad skin extracts on the pain behavior of cancer model mice and its peripheral mechanism of action. Int immunopharmacol. 2017; 42: 90-99. https://goo.gl/L8fdzw
- Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs. 1991; 41: 326-344. https://goo.gl/qjiyp6
- Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996; 52:13-23. https://goo.gl/hm4sKN
- World Health Organization. Guidelines on good agricultural and collection practices (GACP) for medicinal plants. Geneva: 2003. https://goo.gl/tcxGbs
- World Health Organization. Quality controls methods for medicinal plant materials. Geneva; 1998. https://goo.gl/LEWues
- Perry NS, Bollen C, Perry EK and Ballard C. Salvia for dementia therapy: review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav. 2003; 75: 651-9. https://goo.gl/2b4BX6
- Houghton PJ. Ethnopharmacology of some Buddleja species. J. Ethnopharmacol. 1984; 11: 293–308. https://goo.gl/dSR3ZR
- Koster R, Anderson M, De Beer E.J. Acetic acid for analgesic screening. Fed Proc; 1959; 18: 412. https://goo.gl/RwRMrC
- Hunskaar S, Berge OG, Hole K. Dissociation between antinociceptive and antiinflammatory effects of acetylsalicylic acid and indomethacin in the formalin test. Pain. 1986; 25: 125-132. https://goo.gl/ac6kUz
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941; 72: 74-79. https://goo.gl/K6BAXi
- Sahley TL, Berntson GG. Antinociceptive effects of central and systemic administration of nicotine in the rat. Psychopharmacology (Berl). 1979; 65: 279–283. https://goo.gl/RNnukm
- Barros HMT, Tannhauser MAL, Tannhauser SL, Tannhauser M. Enhanced detection of hyperactivity after drug withdrawal with a simple modification of the open-field apparatus. J Pharmacol Methods. 1991; 26: 269–275. https://goo.gl/gFvg9L
- WHO, General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. World Health Organization Switzerland. 2000. https://goo.gl/bXXeTn
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983; 54: 275–287. https://goo.gl/qr6LxS
- Yi T, Zhao ZZ, Yu ZL, Chen HB. Comparison of the anti-inflammatory and anti-nociceptive effects of three medicinal plants known as “Snow Lotus” herb in traditional Uighur and Tibetan medicines. J. Ethnopharmacol. 2010; 128: 405-411. https://goo.gl/BGae3X
- Pinheiro MMG, Miltojevic AB, Radulovic NS, Abdul-Wahab IR, Boylan F, Fernandes P.D. Anti-Inflammatory activity of Choisya ternate Kunth Essential Oil, Ternanthranin, and its two Synthetic Analogs (Methyl and PropylN-Methylanthranilates). PLoS One. 2015; 10: 1012-1063. https://goo.gl/DtTu6d
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51: 5-17. https://goo.gl/ZNdh69
- Simón-Arceo K, González-Trujano E, Coffeen U, Fernández-Mas R, Mercado F, Almanza A, et al. Neuropathic and inflammatory antinociceptive effects and electrocortical changes produced by Salvia divinorum in rats. J. Ethnopharmacol. 2017; 206: 115-124. https://goo.gl/1G6AKT
- Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, et al. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002; 625: 1084–1093. https://goo.gl/JTQYek
- Eisenach JC. Muscarinic-mediated analgesia. Life Sci. 1999; 64: 549–554. https://goo.gl/SaAB93
- Radhakrishnan R, Sluka KA. Spinal muscarinic receptors are activated during low or high frequency TENS-induced antihyperalgesia in rats. Neuropharmacology. 2003; 45: 1111–1119. https://goo.gl/AVbJuf
- Toriyabe M, Omote K, Kawamata T, Namiki A. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesiology. 2004; 101: 983-990. https://goo.gl/h8BCpz
- Freire MA, Guimarães JS, Leal WG, Pereira A. Pain modulation by nitric oxide in the spinal cord. Front neurosci. 2009; 3: 175-181. https://goo.gl/k991nf
- Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV 1 and TRPA 1 mediate peripheral nitric oxide-induced nociception in mice. Plos One. 2009; 4: 75-96. https://goo.gl/3ASJQi
- Millan MJ. Descending control of pain. Prog. Neurobiol. 2002; 66, 355–474. https://goo.gl/H4X8c7
- Bardin L, Bardin M, Lavarenne J, and Eschalier A. Effect of intrathecal serotonin on nociception in rats: influence of the pain test used. Exp. Brain Res. 1997; 113: 81–87. https://goo.gl/jbD9va
- Avila-Rojas SH, Velázquez-Lagunas I, Salinas-Abarca AB, Barragán-Iglesias P, PinedaFarias JB, Granados-Soto V. Role of spinal 5-HT5A, and 5-HT1A/1B/1D, receptors in neuropathic pain induced by spinal nerve ligation in rats. Brain Res. 2015; 1622: 377–385. https://goo.gl/xTHFZU
- Amaya Castellanos E, Pineda Farias JB, Castaneda Corral G, Vidal-Cantu GC, Murbartian J, Rocha Gonzalez HI, et al. Blockade of 5-HT7 receptors reduces tactile allodynia in the rat. Pharmacol. Biochem. Behav. 2011; 99: 591–597. https://goo.gl/6TM9kn
- Miranda HF, Sierralta F, Lux S, Troncoso R, Ciudad N, Zepeda R, et al. Involvement of nitridergic and opioidergic pathways in the antinociception of gabapentin in the orofacial formalin test in mice. Pharmacological Reports. 2015; 67: 399-403. https://goo.gl/Y1sc2e
- Kung AT, Yang X, Li Y, Vasudevan A, Pratt S, Hess P. Prevention versus treatment of intrathecal morphine-induced pruritus with ondansetron. Int J Obstet Anesth. 2014; 23: 222-226. https://goo.gl/z8jq78
- Bermudez Ocana DY, Ambriz Tututi M, Perez Severiano F, Granados Soto V. Pharmacological evidence for the participation of NO–cyclic GMP–PKG–K+ channel pathway in the antiallodynic action of resveratrol. Pharmacology Biochemistry and Behavior. 2006; 84: 535-542. https://goo.gl/m9TNi4

Sign up for Article Alerts