Peripheral Nerve Stimulation and Percutaneous Electrical Nerve Stimulation in Pain Management: A Review and Update on Current Status?
Laura Tyler Perryman*
Stimwave Technologies, Inc. 1310 Park Central Blvd South, Pompano Beach, Florida 33064, USA
*Address for Correspondence: Laura Tyler Perryman, Stimwave Technologies, Inc. 1310 Park Central Blvd South, Pompano Beach, Florida 33064, USA, Tel: 800-965-5134; E-mail: laura@stimwave.com
Submitted: 17 November 2017; Approved: 14 December 2017; Published: 18 December 2017
Citation this article: Perryman LT. Peripheral Nerve Stimulation and Percutaneous Electrical Nerve Stimulation in Pain Management: A Review and Update on Current Status. Int J Pain Relief. 2017;1(1): 036-041.
Copyright: © 2017 Perryman LT. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Wireless Neuromodulation; Peripheral Nerve Stimulation; Peripheral Electrical Nerve Stimulation; Occipital Neuralgia; Headache; Nanomaterials
Download Fulltext PDF
Chronic pain has been a debilitating condition with debilitating consequences on the person and the society. Electrical stimulation of nervous system, currently known appropriately as neuromodulation, has improved the quality of life as well as reducing the opioid medication abuse. Both temporary and permanent neuromodulation therapies have undergone several modifications to improve the acceptance of the methods as well as the efficiency. A Percutaneous Electrical Nerve Stimulation (PENS) is a hybrid short term technique combining transcutaneous electrical nerve stimulation (TENS) and acupuncture utilizing a needle electrode inserted in to soft tissues to modulate the small nerve endings in the soft tissues. Peripheral Nerve Stimulation (PNS) is a standardized technique of neuromodulation that employs the conventional Spinal Cord Stimulation (SCS) components, implanted in the patient’s body. PNS has advanced remarkably well, due to its minimally invasive technique and refined equipment designs, mostly applicable to the conventional SCS. Due to the nature of the equipment not specific to its purpose, PNS suffers from the drawbacks of the bulky SCS implants. The recent advances in wireless technology with nanomaterial components have significantly reduced the adverse events of PNS therapy, since the power generator and its connections are no longer required to be implanted. Encouraging results have been reported with the wireless PNS neuromodulation in cases of occipital neuralgia, craniofacial pain and intercostal post-herpetic neuralgia.
Introduction
Chronic pain is a common symptom necessitating hospital visits. Several medical, non-invasive, minimally invasive and invasive treatments are available implicating the complex nature of pain as an intractable problem. Electrical stimulation of the nervous system, both peripheral and central, has been an accepted therapeutic method with or without breaching the integument. Accordingly transcutaneous and percutaneous stimulation techniques have emerged with remarkable advancements in the technology. Peripheral Nerve Stimulation (PNS) is a reasonably less invasive treatment option compared to stimulation of the central nervous system. Percutaneous Electrical Nerve Stimulation (PENS), by definition implies the route of administration, distinguishing itself from the Transcutaneous Electrical Nerve Stimulation (TENS), and engages the non-neural elements more than the nerve itself. PNS targets a nerve trunk supplying the painful body parts to provide relief.
Nomenclature Of Peripheral Nerve Stimulation
The remarkable work of Ronald Melzak and Patrick Wall in 1965, introduced the theory of pain [1], while William Sweet along with Wall provided the proof of concept of PNS in 1967, when they stimulated their own infraorbital nerve by a needle electrode thereby experiencing the effects of electrical stimulation first hand [2].
Following this, PNS was enthusiastically employed for chronic pain relief but had mixed results mostly due to lack of selection criteria, technical difficulties and failure of systematic application [3]. This explains the distinctly sparse literature on PNS in spite of the awe-inspiring beginning [4].
However, PNS is capable of catering to multiple somatic as well as visceral conditions apart from pain, such as diaphragm palsy, intractable epilepsy, autonomic as well as somatic nerve stimulation for urinary bladder [5,6]. It is important to define PNS, thus, to understand its efficacy.
Peripheral Nerve Stimulation: PNS is electrical stimulation of a specific nerve with a specific name that supplies a very distinct area of body. There is a territory of that nerve and stimulation results in changes in the function of the particular nerve. PNS provides unidirectional paresthesia along that selected peripheral nerve with a better stimulation quality [7]. Accordingly, indication for PNS therapy is neuropathic pain along the nerve distribution so the stimulation is effective along the affected nerve [8]. This can be achieved by open method, wherein the nerve is exposed surgically and the electrodes are placed overlying it or by minimally invasive percutaneous technique.
In case a specific nerve is not stimulated, the procedure is called Peripheral Subcutaneous Field Stimulation (PSFS).
Percutaneous Nerve Stimulation (PENS): A combination of TENS, a surface stimulation method and acupuncture, intradermal needle insertion according to Chinese land marks on the body, is PENS. Acupuncture acts by mechanical stimulation but Electroacupuncture (EA) employs electrical stimulation (2-100 Hz) for analgesic effects mediated through opioid receptors [9,10].
TENS also can be applied with either low (2Hz) or high (50-100 Hz) frequency stimulation on the skin; but not at the same time. At high as well low frequencies TENS produces analgesia by activating smaller motor afferents while high frequency is more selective in stimulating larger diameter a beta afferents to cut down the nociceptor cell activity [11]. PENS delivered via percutaneous insertion of needles in the vicinity of peripheral nerves, however, utilizes both high and low frequencies in a rapidly alternating rhythm to achieve similar effects of stimulation as above [12]. This is particularly useful in patients intolerant to TENS (due to skin irritation or allodynia) and as such avoiding skin resistance, delivers the stimulation to its full potential [13].
Advancements with PNS
The initial pioneering work of Wall, Sweet and Sheldon continued for 20 years with limited expertize and technology [2,14,15]. Difficulties were encountered due to ineffective on and off stimulation method and surgical trauma to the nerves followed by scar tissue formation [8,16,17].
A percutaneous electrical nerve stimulator was developed by Long in 1973, much similar to the indigenous method of Wall and Sweet, using cordotomy electrodes within 18 G needles, initially for PNS screening but later became more of a prototype for PENS [18]. Percutaneous epidural insertion of cylindrical electrode for epidural and PNS had set in the initiative for minimally invasive procedures by Urban and Nashold in 1982 [19]. A simple and less invasive technique introduced by Weiner and Reid for occipital neuralgia [20] improved the confidence in PNS of occipital and trigeminal nerves [21-23] expanding the gamut of indications, implantation methods and type of electrodes. Further safety and simplification of the technique ensued with ultrasound guidance to place to electrodes for stimulation of any named peripheral nerve throughout the body [24,25].Trigeminal and occipital nerves remained to be major nerves to receive PNS for a variety of indications like postherpetic neuralgia, trigeminal neuropathy/neuralgia, migraines and cluster headaches [21,26-32].
The minimally invasive nature of PNS increased the indications to relieve postsurgical pain, low back pain, scapular pain, coccydynia and chronic regional pain syndrome- type 2 by placing the stimulator in close proximity to the peripheral nerve [32-37].
As the popularization of PNS continued, evaluation of outcomes and adverse events started to get attention not only to audit the procedure but to refine the technology also [38,39]. Electrode migrations, fractures, disconnections, erosion of leads and failure of stimulation prompted several modifications [40,41].
Wireless, minimally invasive nano-technology in PNS: Most of the device related complications in PNS arise from its off label application of a conventional Spinal Cord Stimulation (SCS) equipment [42]. The long and bulky SCS device components may not be suitable for the peripheral nerve space and the related complications could be avoided with proper device components.
A miniature Nano electrode system operated by wireless technology is one such advancement in the field of PNS.
The conventional SCS system is bulky with electrodes enclosed in a catheter, attached to long connectors leading to an Implantable Pulse Generator (IPG). All components are placed inside the patient’s body and the system can fail due to malfunction of any of these. Research is ongoing to reduce the bulk of the equipment without compromising the efficiency and IPG life.
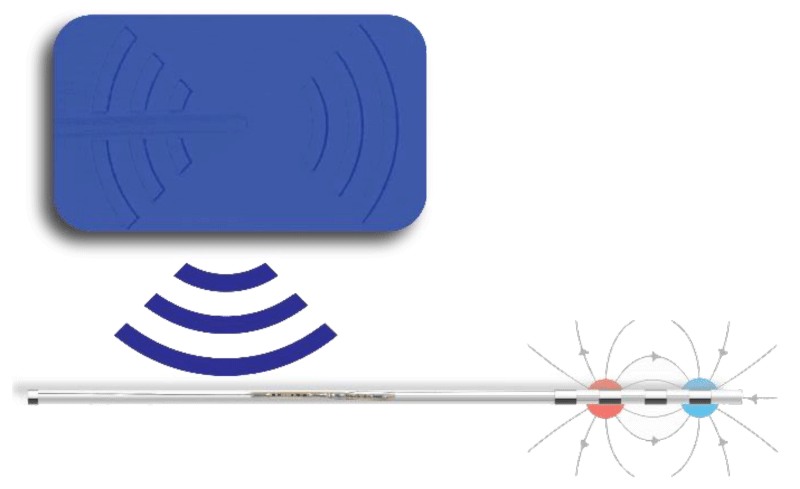
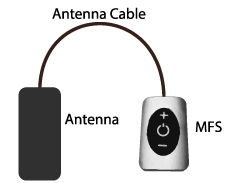
Stimwave device has a novel external Wireless Power Generator (WPG) that uses a dipole antenna for electric field coupling accomplished with very short length pulsed electromagnetic wave at Giga Hertz Frequencies (GHz), ‘microwaves’. This enables miniature implants to be embedded deeper inside the body and also affords minimal loss of power due to the higher frequency at play [43]. The small implants can be successfully placed by percutaneous minimally invasive approaches.
Device description
The stimrelieve® stimulator system (StimRelieve LLC, Fort Lauderdale, FL, USA) implants contain four or eight contacts (3 mm in diameter with 4 mm spacing) and accessed by an implantable electrode contact array, a microprocessor receiver and an antenna embedded within the electrode wire that couples to an external WPG (figures 1 and 2). The implant is passive and maneuvered by the WPG according to the patient-physician protocol (figure 3) required for therapeutic effects.
The spectrum of parameters available for stimulation include amplitude of 1-24 ma, pulse width of 1-1000 msec, and frequency of 1-20,000 hz.
Percutaneous Nerve Stimulation (PENS)
When Wall and Sweet applied temporary electrical stimulation to the infraorbital nerves, they put forth the prototype of PENS [2] similar to the implanted electrodes of Sheldon et al that became models for PNS [14,15]. Placement of electrodes either on the skin or in subcutaneous tissues with TENS, PENS or PNS leads to alterations in blood flow, concentrations of local neurotransmitters and endorphins along with cell membrane polarization thereby inhibiting the nociceptive transmission [44].
For PENS, bipolar needle electrodes are inserted in to tissues for pain relief and removed after the therapy, in conditions like back pain, sciatica, diabetic neuropathy, herpetic neuralgia and headache [45-49]. It combines the simplicity and mechanisms of TENS and EA to stimulate the dermatomal sensory nerve endings to produce analgesia better than TENS and Sham controlled therapy. PENS was shown to reduce consumption of opioids in a systematic randomized study [12,45,49].
Discussion
Chronic pain is a common complaint that takes the general population for a medical visit and is a very prevalent public concern [50,51].
Short term benefits with repetitive administration would be provided by local therapies and topical agents including injectable forms only leading to subsequent failures along with continued suffering. Electrical stimulation of nerves provided better relief with long lasting results but with complex apparatus and power supply as well as variable energy requirements.
PENS, however does not require the complex surgical implantation since the bipolar needle electrode required to stimulate the nerve endings is removed after the therapy and does not demand great technical skills. Selection of the area of stimulation is also not particularly difficult since there is an area of sensory derangement marked out clearly by the patient.
Ghoname et al did a randomized crossover study on PENS to show that the results are superior to TENS in patients with low back pain [45]. Consistently encouraging results with PENS have been published [48,49] for a variety of painful conditions.
However, the sham treatment reported was an issue in its accuracy and as Cummings comments probably reflect the concerns. In his review, Cummings stated that “PENS is neither different in principle nor in practice from EA, and whilst the term accurately reflects the nature of the treatment, there is no substantial justification for referring to PENS as a ‘novel analgesic therapy’ while the term is acceptable for reporting purposes [52].
This might explain why PENS did not become a popular, sustainable neuromodulation approach even though it is less invasive and temporary.
PNS, as a complementary treatment to SCS in nonresponsive cases of neuropathic pain, though was promising in its days of inception, could not meet with the safety requirements, predominantly due to the off-label use of the bulky SCS equipment. PENS (and TENS also) was helpful as a trial stimulation for PNS, along with electrophysiological studies and nerve blocks to make better selection of indications [44,53]. Limited success could only be achieved with the extremity pain, especially in the lower extremities until the morphological configuration of the electrodes was altered to a cylindrical percutaneous type, thus reducing the interface with epineurium and minimizing scar tissue formation [22,54,55]. The modified configuration has improved the access to the sensory afferents in head and face regions as well as extremity peripheral nerves.
Currently available neurostimulator systems, however, have not been designed for use in the peripheral nerve space, and are thus associated with complications and side effects [56,57,58]. Further refinements to the bulky apparatus deemed necessary to make the PNS more acceptable for all its efficacy and applications.
The wireless technology, as described above, due to its inherent design mitigates the negative issues related to these “implantable-only” components of the SCS assembly. Wireless neuromodulation shows promise and paves pathway to expanding number of indications for the relief of chronic pain conditions. A significant reduction in hardware components associated with complications would follow due to the minimally invasive nature of both the technique as well as the technology. A simple lead placement without the need to tunnel and attach an implanted pulse generator can be advantageous to the patient, the surgeon and the health care system in reducing costs, procedure time, postoperative pain, and adverse events while achieving the desired pain control [59]. Stimwave wireless technology has been reported to be safe and effective, as PNS modality in cases of craniofacial pain, occipital neuralgia and postherpetic neuralgia [60-61].
Additionally, the minimally invasive nature of this technology may be offering incomparable benefits to patients with:
1. Compromised immunity as in cases of herpetic neuralgia, retro viral infections, chronic debilitating diseases and malignancy.
2. Comorbid conditions like Diabetes mellitus, chronic renal failure, and anemia.
3. Fragile skin conditions secondary to neuropathy, psoriasis, chronic limb ischemia.
4. Limited life expectancy in painful conditions and those associated with malignancy.
Summary and Perspective
From surface electrical stimulation with TENS to implantation of electrodes and power generators, pain management has progressed to a minimally invasive therapy with significant improvement in disability. PENS and AE in spite of their ease of application have not been popular in neuromodulation most likely due to the lack of evidence based literature. PNS on the other hand found increasing acceptance as a preferred method to control intractable pain following the percutaneous technique. However, the technology, being an off-label use of SCS, required finesse and further advancements in terms of its energy delivery as well reduced bulk of implanted material. Wireless neuromodulation with nanomaterials provide the required technological substrate missing in application so far. Initial experience in cases with refractory occipital neuralgia, craniofacial pain and intercostal neuralgia due to herpes zoster have been encouraging. Further experience in larger groups of patients would be expected to make this wireless stimulation technology to replace the bulky, cumbersome implantation devices in the limited peripheral nerve space.
- Melzack RA, Wall PD. Pain mechanisms: a new theory. Science. 1965; 150: 971-979. https://goo.gl/srReze
- Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967; 155: 108-109. https://goo.gl/3XjceK
- Stanton Hicks M. Peripheral nerve stimulation for pain: peripheral neuralgia and complex regional pain syndrome; in Krames ES, Peckham PH, Rezai AR: Neuromodulation. London, Elsevier. 2009; 397- 409.
- Gybels JM, Nuttin BJ. Peripheral nerve stimulation; in Loeser J: Bonica’s Management of Pain, ed 3. Philadelphia, Lippincott. 2000; 1851- 1855.
- Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008; 5: 100-106. https://goo.gl/8bnRq2
- Stuart RM, Winfree CJ. Neurostimulation technique for painful peripheral nerve disorders. Neurosug Clin N Am. 2009; 20: 111- 120. https://goo.gl/aqF2w4
- Abejon D, Perez-Cajaraville J. Peripheral nerve stimulation: Definition. In Slavin KV: Peripheral nerve stimulation. Prog Neurol Surg. Basel, Karger, 2011; 24: 203-209.
- Law JD, Sweet J, Kirsch WM. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J Neurosurg. 1998; 52: 482- 485. https://goo.gl/JSmJLt
- Goldstein A, Naidu A. Multiple opioid receptors: Ligand selectivity profiles and binding site signature. Mol Pharmacol. 1989; 36: 265-272. https://goo.gl/yXHUc9
- Sun SL, Han JS. High and low frequency electro acupuncture analgesia are mediated by different types of opioid receptors at spinal level: A cross tolerance study. Sheng Li Xue Bao. 1989; 41: 416-20. https://goo.gl/hr4VS6
- Johnson MI. Transcutaneous electrical nerve stimulation. In: Kitchen S, ed. Electrotherapy: Evidence Based Practice. Edinburgh: Churchill Livingstone; 2002: 259-86.
- Ghoname ES, Craig WF, White PF, Ahmed HE, Hamza MA, Gajraj NM, et al. The effect of stimulus frequency on the analgesic response to percutaneous electrical nerve stimulation in patients with chronic low back pain. Anesth Analg. 1999; 88: 841-6. https://goo.gl/53XXSx
- Raphael JH, Raheem TA, Southall JL, Bennett A, Ashford RL, Williams S. Randomized double-blind sham-controlled crossover study of short-term effect of percutaneous electrical nerve stimulation in neuropathic pain. Pain Med. 2011; 12: 1515-22. https://goo.gl/mJS9U9
- Shelden CH: Depolarization in the treatment of trigeminal neuralgia: evaluation of compression and electrical methods; clinical concept of neurophysiological mechanism; in Knighton RS, Dumke PR: Pain. Boston, Little, Brown. 1966; 373-386.
- Shelden CH, Paul F, Jacques DB, Pudenz RH: Electrical stimulation of the nervous system. Surg Neurol. 1975; 4: 127-132. https://goo.gl/oNHecz
- Kirsch WM, Lewis JA, Simon RH: Experiences with electrical stimulation devices for the control of chronic pain. Med Instrum. 1975; 9: 217-220. https://goo.gl/2c9PFH
- Nielson KD, Watts C, Clark WK. Peripheral nerve injury from implantation of chronic stimulating electrodes for pain control. Surg Neurol. 1976; 5: 51-53. https://goo.gl/AHSy6p
- Long DM. Electrical stimulation for relief of pain from chronic nerve injury. J Neurosurg. 1973; 39: 718-722. https://goo.gl/258BZh
- Urban BJ, Nashold BS Jr. Combined epidural and peripheral nerve stimulation for relief of pain. Description of technique and preliminary results. J Neurosurg. 1982; 57: 365-369. https://goo.gl/6BYQ29
- Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999; 2: 217-221. https://goo.gl/kcgz3v
- Slavin KV, Burchiel KJ. Use of long-term nerve stimulation with implanted electrodes in the treatment of intractable craniofacial pain. J Neurosurg. 2000; 92: 576.
- Weiner RL, Alo KM, Reed KL, Fuller ML. Subcutaneous neurostimulation for intractable C-2-mediated headaches. J Neurosurg. 2001; 94: 398.
- Dunteman E. Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation. 2002; 5: 32-37. https://goo.gl/Hehb6r
- Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009; 10: 1369-77. https://goo.gl/D6v4ar
- Peterson EA, Slavin KV. Peripheral nerve/field stimulation for chronic pain. Neurosurg Clin N Am. 2014; 25: 789-97. https://goo.gl/oKABuP
- Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004; 55: 135-141. https://goo.gl/AiERMW
- Slavin KV, Wess C. Trigeminal branch stimulation for intractable neuropathic pain: technical note. Neuromodulation. 2005; 8: 7-13. https://goo.gl/Nr8SGP
- Amin S, Buvanendran A, Park KS, Kroin JS, Moric M. Peripheral nerve stimulator for the treatment of supraorbital neuralgia: a retrospective case series. Cephalalgia. 2008; 28: 355-359. https://goo.gl/KQbkh3
- Reed KL, Black SB, Banta CJ II, Will KR. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. 2010; 30: 260-271. https://goo.gl/XcFp87
- Burns B. ‘Dual’ occipital and supraorbital nerve stimulation for primary headache. Cephalalgia. 2010; 30: 257-259. https://goo.gl/vdaaGd
- Narouze SN, Kapural L. Supraorbital nerve electric stimulation for the treatment of intractable chronic cluster headache: a case report. Headache. 2007; 47: 1100-1102. https://goo.gl/QNmQx8
- Stinson LW Jr, Roderer GT, Cross NE, Davis BE. Peripheral subcutaneous Electrostimulation for control of intractable post-operative inguinal pain: a case report series. Neuromodulation. 2001; 4: 99-104. https://goo.gl/Nf6k7R
- Paicius RM, Bernstein CA, Lempert Cohen C. Peripheral nerve field stimulation for the treatment of chronic low back pain: preliminary results of long-term follow-up: a case series. Neuromodulation. 2007; 10: 279-290. https://goo.gl/ZrLDYG
- Theodosiadis P, Samoladas E, Grosomanidis V, Goroszeniuk T, Kothari S: A case of successful treatment of neuropathic pain after a scapular fracture using subcutaneous targeted neuromodulation. Neuromodulation 2008; 11: 62-65. https://goo.gl/WcnwEz
- Kothari S. Neuromodulatory approaches to chronic pelvic pain and coccygodynia. Acta Neurochir Suppl. 2007; 97: 365-371. https://goo.gl/WgdJXT
- Monti E. Peripheral nerve stimulation: a percutaneous minimally invasive approach. Neuromodulation 2004; 7: 193-196. https://goo.gl/5Zffiq
- Falowski S, Wang D, Sabesan A, Sharan A. Occipital nerve stimulator systems: review of complications and surgical techniques. Neuromodulation. 2010; 13: 121-125. https://goo.gl/hQPjsq
- Jasper JF, Hayek SM. Implanted occipital nerve stimulators. Pain Physician. 2008; 11: 187-200. https://goo.gl/BfGgyJ
- Gofeld M. Anchoring of suboccipital lead: case report and technical note. Pain Pract. 2004; 4: 307-309. https://goo.gl/cnQTvU
- Trentman TL, Slavin KV, Freeman JA, Zimmerman RS. Occipital nerve stimulator placement via a retromastoid to infraclavicular approach: a technical report. Stereotact Funct Neurosurg. 2010; 88: 121-125. https://goo.gl/eGbaVM
- Eldabe S, Buchser E, Duarte R. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: A review of the literature. Pain Med. 2016; 17: 325-336. https://goo.gl/qkSJzH
- Yearwood TL, Perryman LT Peripheral neurostimulation with a microsize wireless stimulator. In. Slavin (Ed) Stimulation of the peripheral nervous system. The Neuromodulation Frontier. Prog Neurol Surg. Basel, Karger. 2016; 29: 168-191. https://goo.gl/2nNQoX
- Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976; 45: 692- 699. https://goo.gl/4G9BrL
- Ghoname EA, Craig WF, White PF, Ahmed HE, Hamza MA, Henderson BN, et al. Percutaneous electrical nerve stimulation for low back pain: a randomized crossover study. JAMA. 1999; 281: 818-823. https://goo.gl/6t9HSU
- Weiner DK, Perera S, Rudy TE, Glick RM, Shenoy S, Delitto A. Efficacy of percutaneous electrical nerve stimulation and therapeutic exercise for older adults with chronic low back pain: a randomized controlled trial. Pain. 2008; 140: 344-357. https://goo.gl/GJEjik
- Hamza MA, White PF, Craig WF, Ghoname ES, Ahmed HE, Proctor TJ, et al. Percutaneous electrical nerve stimulation: a novel analgesic therapy for diabetic neuropathic pain. Diabetes Care. 2000; 23: 365-370. https://goo.gl/tgmPHy
- Ahmed HE, White PF, Craig WF, Hamza MA, Ghoname ES, Gajraj NM. Use of percutaneous electrical nerve stimulation (PENS) in the short-term management of headache. Headache. 2000; 40: 311-315. https://goo.gl/VtEMR8
- Ahmed HE, Craig WF, White PF Ghoname ES, Hamza MA, Gajraj NM, et al. Percutaneous electrical nerve stimulation: an alternative to antiviral drugs for acute herpes zoster. Anesth Analg. 1998; 87: 911-914. https://goo.gl/AiV12H
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002; 23: 131-137. https://goo.gl/bqD8Gs
- Mäntyselkä P1, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001; 89: 175-180. https://goo.gl/H3iRTY
- Cummings M. Percutaneous electrical nerve stimulation-electroacupuncture by another name? A comparative review. Acupunct Med. 2001; 19: 32-35. https://goo.gl/Y8RvEB
- Nashold BS Jr, Mullen JB, Avery R. Peripheral nerve stimulation for pain relief using a multicontact electrode system: technical note. J Neurosurg. 1979; 51: 872- 873. https://goo.gl/dZpMRU
- Popeney CA, Alo KM. C1- 2-3 peripheral nerve stimulation (PNS) for the treatment of disability associated with transformed migraine. Headache. 2003; 43: 369-373. https://goo.gl/EnK4Vv
- Oh MY, Ortega J, Bellotte JB, Whiting DM, Alo KM. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a C1- 2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004; 7: 103-112. https://goo.gl/wMxq9j
- Parker JL, Cameron T. Technology for Peripheral Nerve Stimulation Prog Neurol Surg 29: 1-19, 2015. https://goo.gl/VbMmKN
- Schwedt TJ, Dodick DW, Hentz J, Trentman TL, Zimmerman RS. Occipital nerve stimulation for chronic headache-long-term safety and efficacy. Cephalalgia. 2007; 27: 153-157. https://goo.gl/xKK4S8
- Slavin KV. Technical aspects of peripheral nerve stimulation: hardware and complications. Prog Neurol Surg. 2011; 24: 189-202. https://goo.gl/XbP1nx
- Yearwood TL, Perryman LT. Peripheral Neurostimulation with a Microsize Wireless Stimulator. Prog Neurol Surg. 2015; 29: 168-191. https://goo.gl/SpWUbs
- Weiner RL, Garcia CM, Vanquathem N. A novel miniature wireless neurostimulator in the management of chronic craniofacial pain: preliminary results from a prospective pilot study. Scand J Pain. 2017. https://goo.gl/QDiL3J
- Perryman LT, Speck B, Weiner RL. A novel wireless minimally invasive neuromodulation device for the treatment of chronic intractable occipital neuralgia: case illustrations. J Neurol Stroke. 2017; 6: 213. https://goo.gl/BkRdzK
- Billet B, Wynendaele R, Vanquathem N. A novel minimally invasive wireless technology for neuromodulation via percutaneous intercostal nerve stimulation (PNS) for post-herpetic neuralgia: A case report with short term follow up. Pain Pract. 2017. https://goo.gl/ENYYCe


Sign up for Article Alerts