Comparison of Postoperative Analgesia between Neostigmine and Dexamethasone as an Adjuvant to Ropivacaine (0.25%) in Caudal Block Administered to Children under Sonographic Guidance?
Kunal KS1* and Rajesh KV2
1Department of Neuro-Anaesthesia and Neuro Critical care, NIMHAMS, Bengaluru, Karnataka, India
2Department of Anaesthesiology, IGMC Shimla, Himachal Pradesh, India
*Address for Correspondence: Kunal KS, Department of Neuro-Anaesthesia and Neuro Critical care, Old KABINI hostel, NIMHAMS, Bengaluru, India, Postal code: 560029, Tel: +701-838-8348; E-mail: kunaal_kumar@yahoo.com
Submitted: 07 June 2019; Approved: 19 July 2019; Published: 20 July 2019
Citation this article: Kunal KS, Rajesh KV. Comparison of Postoperative Analgesia between Neostigmine and Dexamethasone as an Adjuvant to Ropivacaine (0.25%) in Caudal Block Administered to Children under Sonographic Guidance. Int J Pain Relief. 2019;3(1): 001-005.
Copyright: © 2019 Kunal KS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Introduction
A child also feels pain as an adult, therefore it is imperative to ensure adequate post-operative analgesia in children undergoing surgical procedures. Postoperative intravenous use of opioids and NSAIDS is associated with serious adverse effects in the tender pediatric age. With the advent of sonography in the past few decades, regional anesthesia in infants and pediatric patients has undergone a revolution. Intraoperative need of inhalational or intravenous anesthetics is reduced by concomitant administration of caudal block. Sonoanatomical landmarks aid in administration of caudal block by direct visualization of penetration of sacro-cocygeal ligament by the echogenic needle (EchoStimTM). This has dramatically reduced the failure rates of caudal block associated with classical technique. A single shot caudal block provides analgesia for 2- 4 hours only [1]. Therefore addition of adjuvants to caudal block has been a focus of research for past two decades which has involved the use of morphine, fentanyl, ketamine, clonidine, dexmedetomidine, midazolam and even adrenaline [2-6]. However, the quest to find an ideal adjuvant for caudal block still remains. Neostigmine has been used as an adjuvant for caudal block in varying doses from 2 to 50 µg/ kg [7,8]. It provides pain relief by preventing the breakdown of acetylcholine in spinal cord mediated by spinal muscarinic (M1) receptors, thereby resulting in prolongation of analgesia. The main adverse effect ascribed with epidural neostigmine is nausea and vomiting, but it is seen at dose ranges of 20-50 µg/ kg. Therefore we decided to use low dose of 1 µg/ kg of neostigmine in our study group. Preservative free dexamethasone, on the other hand, has emerged as a promising adjuvant for caudal block, being bereft of adverse effects pertaining to the anticholinergic and opioid groups of adjuvants. The prolongation of analgesia by dexamethasone is suspected to be mediated by the inhibition of synthesis and release of various inflammatory mediators. Dexamethasone also has local vasoconstrictive effect on glucocorticoid receptors which results in reduced local anaesthetic absorption, thereby prolonging of the duration of block. Dexamethasone produces analgesia by blocking transmission of nociceptive myelinated c fibers and suppressing ectopic neuronal discharge. Dexamethasone also inhibits the action of phospholipase A and it alters the function of potassium channels in the excitable cells by via glucocorticoid receptors. Neuroprotective role of dexamethasone has been attributed to its effects by the threonine-serine protein kinase B-dependent mechanism. Therefore we decided to study the adjuvant properties of this drug in our research work. The primary objective of our study was to compare the postoperative analgesia after USG guided caudal block by administering dexamethasone or neostigmine as an adjuvant to ropivacaine. Secondary objectives were to evaluate postoperative analgesic consumption and monitor for adverse effects.
Material and Methods
This study was approved by the research and ethical committee of IGMC Shimla. Patients to be studied were divided into three equal groups of 25 patients each, i.e CCB (Control), NCB (Neostigmine) and DCB (Dexamethasone). Randomization was done by random computer generated numbers. One anaesthetist prepared the drugs and was present at the area of randomizing the patients. The anaesthetist who was administering the caudal block was blinded to the contents of the injectant mixture. The intraoperative monitoring and postoperative monitoring was done by the anesthetist who was unaware of the content of injectate mixture given to the patient. The inclusion criteria comprised of patient of ASA-I physical status who were scheduled for infraumblical and lower limb surgeries. The exclusion criteria were: local infection in the caudal area; history of bleeding disorder; allergy to local anaesthetics; pre-existing neurological disorders and contraindication for regional block. All the patients were pre-medicated with midazolam syrup 0.25 mg/ kg. Venous access was secured in dorsum vein of hand after prior application of prilocaine cream. The monitors were attached for continuous ECG monitoring, NIBP, EtCO2 and SpO2 monitoring. Induction of anesthesia was done with propofol 2 mg/ kg iv and airway was secured with supraglottic airway device IgelTM of appropriate size. Maintenance of anaesthesia was carried out by oxygen:nitrous mixture of 50:50 % alongwith sevoflurane. The patient was now positioned in lateral posture, and the overlying skin was cleaned and draped under aseptic precautions. Caudal block was administered under sonographic guidance using the injectate mixture as per the respective study group. Group CCB received only ropivacaine (0.25%) 0.9 ml/kg. Group DCB received ropivacaine (0.25%) 0.9 ml/ kg and dexamethasone 0.1 mg/ kg, whereas group NCB received ropivacaine (0.25%) 0.9 ml/ kg and neostigmine 1 µg/ kg. Such low dose of neostigmine was chosen because as per the studies conducted by Karaaslan et al. [9] the higher doses of neostigmine offered no additional benefit of extension of duration of analgesia. The 6-13 MHz transducer of Micromax SonositeTM ultrasound machine was used to obtain the view of sacral hiatus. The 21G (5 cm long) EchoStimTM needle with short 30° block bevel was inserted via in-plane technique through sacral hiatus. Once the needle pierced the sacrococcygeal ligament, the needle tip disappeared due to acoustic shadow. After prior negative aspiration the injectate was administered at a speed of 0.5 ml/ sec and its spread was confirmed by real time visualization of anterior displacement of dura. The patient was now turned supine and surgery was allowed. Intraoperative vitals were monitored and maintained. At the end of the surgical procedure the patient was extubated and shifted to PACU. The efficacy of caudal block was evaluated by the post-operative severity of pain and the time of demand of first rescue analgesia (paracetamol 10 mg/ kg iv). The patients were monitored at post-anaesthetic care unit. The postoperative pain was evaluated by EVENDOL (Evaluation Enfant Douleur) scale because of higher validation criterias and excellent inter- evaluator reliability when compared to other scales like FLACC, CHEOPS, TPPPS etc. The EVENDOL score is independent of anxiety and tiredness level of the child because of its unique evaluation method. The details about this scale are given in the table 1. Patients with mean score greater than or equal to 4 were administered paracetamol 10 mg/ kg iv. This therapeutic score took into consideration the value recorded at the time of examining the operated area of the child. The sample size was calculated using Power Analysis and Sample Size (PASS) software. Statistical analysis was done by IBM-SPSS software using ANOVA and post hoc analysis was done by Tukey test.
Results
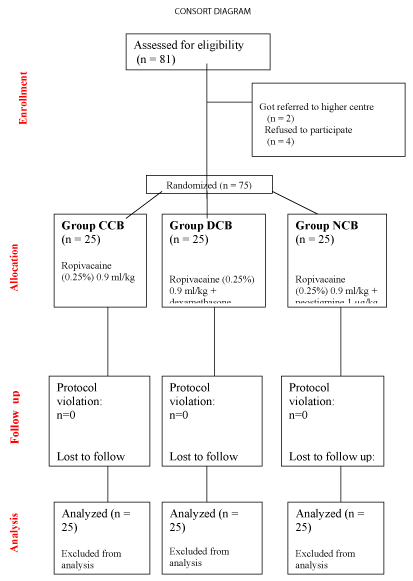
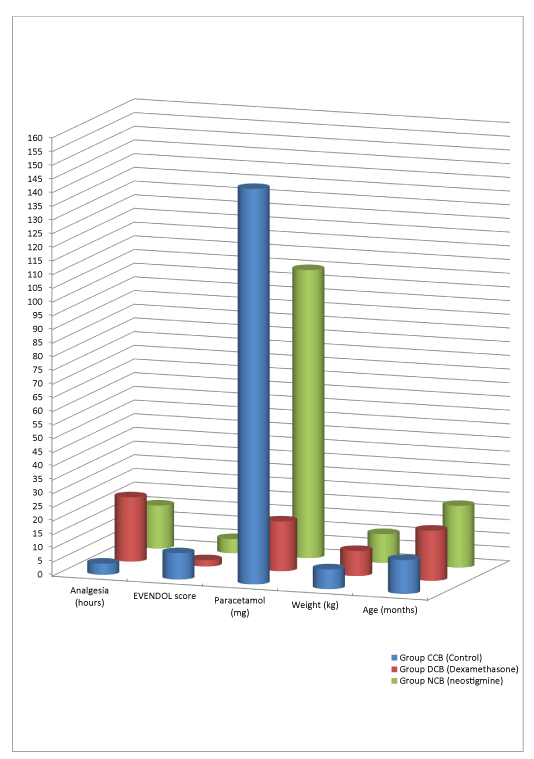
The demographic characteristics of the patients were recorded. After a validated an informed consent obtained from the parent of the child, a total 81 patients of either sex, from age 2 month to 4 years undergoing infra-umbilical surgeries like clubfoot correction, hernia repair etc. were enrolled. The parents of 4 patients refused to give consent for participation in the study, while two patients got referred to higher institute due to complicated surgical pathology. The remaining 75 patients were assigned into three equal groups of 25 patients each, i.e CCB (control), NCB (neostigmine) and DCB (dexamethasone) (Figure 1). The mean duration of postoperative analgesia in group CCB was 244.2 + 26.915 min, in group DCB was 1410.84 + 37.7543 min, whereas in group NCB was 943.12 + 32.5376 min. (p -value < 0.0001). The MAP, HR, RR and SpO2 remained stable throughout the surgical procedure and during the study period. The mean EVENDOL score at the time of first requirement of rescue analgesic was 9.64 + 2.0388 in group CCB, 2.28 + 1.5684 in group DCB and 5.2 + 0.9574 in group NCB (p < 0.0001). The mean postoperative rescue analgesic consumption of paracetamol in group CCB was 144.4 + 52.6054 mg, whereas in group DCB it was 18.2 + 39.8403 mg, while in group NCB it was 105.2 + 24.811 mg (p-value < 0.0001). No incidence of PONV was seen in the DCB group of patients, while 1 patient of group NCB and 2 patients of group CCB had a single episode vomiting after surgery. However there was no incidence of post-operative pulmonary complications like pneumonitis or respiratory depression in any patients of our study group (Table 2).
Discussion
Postoperative pain is associated with detrimental effects on hemodynamic status, anxiety and postoperative healing. Caudal block ensures adequate management of postoperative analgesia in pediatric patients undergoing infra-umbilical and lower limb surgeries. Sonography has greatly affected the success rate of caudal blocks and avoids the multiple needle pricks as seen with blind caudal blocks. The prediction of depth of space helps in determining the trajectory for needle insertion and has therefore improved the success rate in caudal blocks. Bogin and Stulin [10] were the first to report use of ultrasound for central neuraxial blocks. Cork et al. [11] were the first group of anesthesiologists to define relevant anatomical landmarks for epidural anesthesia. Chen et al. [12] described caudal blocks using sonographic imaging. Their study cohort had a mean diameter of sacral canal as 5.3 + 2 mm at the sacral hiatus, whereas the distance between sacral cornu was 9.7 + 1.9 mm. Also findings like closed sacral hiatus and narrow sacral hiatus (1.5 mm) were also observed in their study (Figure 2). All the patients in our study had normal sacral hiatus anatomy. Triffterer et al. [13] studied 50 patients aged up to 6 years undergoing caudal block being administered ropivacine (0.2% or 0.35% ) 1 ml/kg at different speeds ( 0.25 ml/ sec and 0.5 ml/ sec) and found out that ultrasound guided caudal block is a safe technique for analgesia in pediatric patients. Kim et al. [14] conducted a double blinded study on 80 children aged 6 months to 5 years who underwent orchiopexy, using 1.5 ml/ kg ropivacaine (0.15%) with or without 0.1 mg/ kg dexamethasone. They used FLACC and CHEOPS scale for pain assessment and found that the dexamethasone group patients were pain free for 48 hours. These results correlate with the DCB group of our study where the mean analgesia period lasted for 1410.84 + 37.7543 minutes (i.e 23.51 hours). The difference of longer duration of analgesia in study by Kim et al is probably due to use of larger volume of injectant mixture i.e 1.5 ml/ kg used in their study, when compared to our injectant volume of 0.9 ml/kg. Also this difference could have arisen due to the variation of pain evaluation scale between our study and the study by Kim et al. There were no incidence of adverse effects in the DCB group of our study. Attardi et al. [15] showed that dexamethasone causes decreased nociceptive C-fibre activity via a direct effect on glucocorticoid receptors and inhibition of potassium channels. Shishido et al. [16] suggested that by local vasoconstrictive effect there occurs a reduction of local anaesthetic absorption, which leads to quicker onset and prolongation of blockade. Stan et al. [17] showed that glucocorticoids can prolong analgesia period by suppressing the synthesis of inflammatory mediators. Therefore these mechanisms exert a cumulative effect for the benefit of patient by prolonging analgesia duration and suppressing postoperative surgical would inflammation, which shall aid in earlier healing. El-Feky et al. [18] conducted a comparative study between fentanyl, dexmedetomidine, dexamethasone as adjuvant to local anesthetics in caudal block and found lower postoperative pain score in dexamethasone group in comparison to fentanyl group. They however used MOPS score to assess the pain score after caudal block, and there were incidence of vomiting in the dexamethasone group. However there was no incidence of vomiting in our study group patients who were given dexamethasone. Turan et al. [19] conducted a study of neostigmine 2 µg/ kg as adjuvant to ropivacaine (0.25%) in caudal block on children undergoing inguinal hernia and hypospadiasis surgery. It was observed that the first requirement of analgesia was around 19.2 + 5.5 hours. Batarseh et al. [20] conducted a study of different doses of neostigmine in caudal block with bupivacaine 0.25% and found that 1.5 µg/kg neostigmine, 3 µg/ kg neostigmine and 6 µg/ kg neostigmine produced mean analgesia duration of 16.35 hours, 16.8 hours and 16.65 hours in the respective groups. The mean duration of analgesia in our group is 943.12 + 32.537 minutes (i.e 15.71 hours) which can be attributed to the use of an even lower dose of neostigmine i.e 1 µg/ kg in our patients.
No additional group concerning study of parenteral dexamethasone was studied in our research. This was because as per the study by Srinivasan et al. [21] the mean time of requirement of first rescue analgesia in caudally administered dexamethasone group was 720 minutes whereas it was 620 minutes in the parenterally administered dexamethasone group (p < 0.001). This clearly demonstrated the superiority of caudally administered dexamethasone with respect to parenteral dexamethasone. Therefore we decided only to compare the caudally administered adjuvants in our study.
When comparing the two drugs under our study we would like to conclude that dexamethasone is a better adjuvant to neostigmine in caudal blocks because its use lowers postoperative analgesic consumption, enhances the duration of analgesia and has minimal adverse effects, The markedly decreased requirement of postoperative analgesic consumption associated in the group DCB (18.2 + 39.840 mg), favors the use of dexamethasone over neostigmine (105.2 + 24.811) as an adjuvant.
Limitations
We did not measure the serum concentration and CSF levels of the adjuvants used in our study in order to quantify the systemic absorption of these drugs (if any). The administration of general anaesthesia prior to caudal block was a confounding variable in assessment of analgesia.
- Dalens B, Hasnaoui A. Caudal Anaesthesia in paediatric surgery: success rate and adverse effects in 750 consecutive patients. Anesth Analg. 1989; 68: 83-99. https://bit.ly/2YeUOhV
- Wolf AR, Hughes D, Wade A, Mather SJ, Prys-Roberts C. Postoperative analgesia after paediatric orchidopexy: evaluation of a bupivacaine-smorphine mixture. Br J Anaesth. 1990; 64: 430-435. https://bit.ly/2GhfvPW
- Ahuja S, Yadav S, Joshi N, Chaudhary S, Madhu SV. Efficacy of caudal fentanyl and ketamine on post-operative pain and neuroendocrine stress response in children undergoing infraumbilical andperineal surgery: a pilot study. J Anaesthesiol Clin Pharmacol. 2015; 31: 104-109. https://bit.ly/32BjgJL
- Cook B, Grubb DJ, Aldridge LA, Doyle E. Comparison of the effects of adrenaline, clonidine and ketamine on the duration of caudal analgesia produced by bupivavaine in children. Br J Anaesth. 1995; 75: 698-701. https://bit.ly/2LsvGyb
- Naguib M, Sharif AMY, Seraj M, EI Gammal M, Dawlatly AA. Ketamine for caudal analgesia in children-Comparison with caudal bupivacaine. Br J Anaesth. 1991; 67: 559-564. https://bit.ly/2Sn57Lo
- Gupta S, Pratap V. Addition of Clonidine or Dexmedetomidine to Ropivacaine prolongs caudal analgesia in children. Indian J Pain. 2014; 28: 36-41.
- Batra YK, Arya VK, Mahajan R, Chari P. Dose response study of caudal neostigmine for post-operative analgesia in paediatric patients undergoing genitourinary surgery. Paediatr Anaesth. 2003; 13: 515-521. https://bit.ly/2JDhnVn
- Abdulatif M, Sanabary M. Caudal neostigmine, bupivacaine and their combination for postoperative pain management after hypospidias surgery in children. Anesth Analg. 2002; 95: 1215-1218. https://bit.ly/2JFkNqB
- Karaaslan K, Gulcu N, Ozturk H, Sarpkaya A, Colak C, Kocoglu H. Two different doses of caudal neostigmine co-administered with levobupivacaine produces analgesia in children. Pediatric Anesthesia. 2009; 19: 487-493. https://bit.ly/2O4OqpI
- Bogin IN, Stulin ID. Application of the method of 2-dimensional echo spondylography for determining landmarks in lumbar punctures. Zh Nevropatol Psikhiatr Im S S Korsakova. 1971; 71: 1810-1811.
- Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980; 52: 513-516. https://bit.ly/2JSskkG
- Chen CP, Tang SF, Hsu TC, Tsai WC, Liu HP, Chen MJ, et al. Ultrasound guidance in caudal epidural needle placement. Anesthesiology. 2004; 101: 181-184. https://bit.ly/2XRYw1L
- Triffterer L, Machata AM, Latzke D, Willschke H, Rebhandl W, Kimberger O, et al. Ultrasound assessment of cranial spread during caudal blockade in children: effect of the speed of injection of local anaesthetics. Br J Anaesth. 2012; 108: 670-674. https://bit.ly/2XXUsId
- Kim EM, Lee JR, Koo BN, Im YJ, Oh HJ, Lee JH. Analgesic efficacy of caudal dexamethasone combined with ropivacaine in children undergoing orchiopexy. Br J Anaesth. 2014; 112: 885-891. https://bit.ly/2XWhmzS
- Attardi B, Takimoto K, Gealy R, Severns C, Levitan ES: Glucocorticoid induced upregulation of a pituitary K+ channel mRNA in vitro and in vivo. Receptors Channels. 1993; 1: 287-293. https://bit.ly/2StDRLl
- Shishido H, Kikuchi S, Heckman H, Myers RR. Dexamethasone decreases blood flow in normal nerves and dorsal root ganglia. Spine. 2002; 27: 581-586. https://bit.ly/2YgaeCx
- Stan T, Goodman EJ, Bravo-Fernandez C, Holbrook CR. Adding methylprednisolone to local anaesthetic increases the duration of axillary block. Reg Anaesth Pain Med. 2004; 29: 380-381. https://bit.ly/2LVd0GS
- Elham MEl-Feky, Ahmed AAbd El Aziz. Fentanyl, dexmedetomidine, dexamethasone as adjuvant to local anesthetics in caudal analgesia in pediatrics: A comparative study. Egyptian Journal of Anaesthesia. 2015; 31: 175–180. https://bit.ly/2JHbFC5
- Turan A, Memis D, Basaran UN, Karamanlioglu B, Sut N. Caudal ropivacaine and neostigmine in pediatric surgery. Anesthesiology 2003; 98: 719-712. https://bit.ly/2O2Ai09
- Batarseh E, Majali Z, Nsour N, Daaja M, Nabukhotna O. Caudal bupivacaine-neostigmine effect on postoperative pain relief in children. JRMS March. 2015; 22: 24-29. https://bit.ly/2YeXmfZ
- Srinivasan B, Karnawat R, Mohammed S, Chaudhary B, Ratnawat A, Kothari SK. Comparison of caudal and intravenous dexamethasone as adjuvants for caudal epidural block: A double blinded randomised controlled trial. Indian J Anaesth. 2016; 60: 948-954. https://bit.ly/30L6Xsn

Sign up for Article Alerts