Sympathetic Ganglion Block and Neridronate Infusion for Early Phase CPRS-I Treatment: Effects on Pain and on Microcirculation?
Giuseppe Gagliardi1* and Francesco Ceccherelli2
1Departement of anaesthesia and intesive care, AULSS 5 Veneto, Italy
2AIRAS – Padua Italy
*Address for Correspondence: Giuseppe Gagliardi, Departement of anaesthesia and intesive care, AULSS 5 Veneto, Italy, Tel:+390426940828; Fax: +390426940564; E-mail: giuseppe.gagliardi@aulss5.veneto.it
Submitted: 20 August 2019; Approved: 21 October 2019; Published: 22 October 2019
Citation this article: Gagliardi G, Ceccherelli F. Sympathetic Ganglion Block and Neridronate Infusion for Early Phase CPRS-I Treatment: Effects on Pain and on Microcirculation. Int J Pain Relief. 2019;3(1): 006-011.
Copyright: © 2019 Gagliardi G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Pain; CPRS 1; Sympathetic nerve block
Download Fulltext PDF
CRPS can be describedas a painful inflammatory conditions that occurs in most cases after a traumatic lesion, suchassprain or fracture. The main physiopathological elements involved in the genesis of CPRS-I are essentially: autonomic nervous system activity, neurogenic inflammation and micro vascularimpairment. The aim of the studyis to investigate the effect of an integrated treatment, which consists of an anesthetic block of the sympathetic lumbarchain and a neridronatein fusion protocol, on pain and micro circulatory variations in patient saffected by CPRS of lower limb.
Twelve patient saffected by early stage CPRS-I with a duration of pain of 3 ± 1,2 months havebeen enrolled. They havebeen treated with a neridronate infusion of 100 mg x 4 times over 10 days; and simultaneously, theyunderwent an ipsilateral lumbar sympathetic nerveblock.
Painintensity, function restoration and analisys of microcirculation using the LDF (laser doppler flowmeter) havebeen monitored.
The results have shown a quick clinical improvement, with a reduction in pain severity and a restoration of micro circulation function.
The association of neridornate infusion and sympathetic nerve block has been found to be effective and safe for early stage CPRS-I treatment.
Introduction
CRPS can be described as a painful inflammatory condition which occurs in most cases after a traumatic lesion, such as a sprain or fracture. The International Association or the Study of Pain (IASP) has classified CPRS in two different types. CPRS-I does not have any sign of nervous impairment and includes most patients with a CPRS diagnosis, whereas CPRS-II is complicated by a nervous impairment [1].
The main physio pathological elements involved in genesis of CPRS-1 are essentially: auto nomical nervous system activity, neurogenic inflammation and micro vascular impairment. Formerly, sympathetic hyperactivity had been considered the main cause of the origin and of the persistance of CPRS-I, so that sympathetically maintained pain was considered to be a synonym of it [2]. During the following years, that opinion has been changing, but the sympathetic nervous system is in some way involved with the pathophysiology The expression of α1-adrenoceptor mRNA is up regulated in DRG neurons after peripheral nerve injury or inflammation typical of what is seen in CRPS type I; and an increase in α1-adrenoceptors is observed in hyperalgesic skin of patients affected [3].
Recent data have shown that other mechanisms, such as neurogenic inflammation and central sensitisation, could well be responsible for all symptoms [4].
In the early stage, CRPS patients are associated with an increase in pro inflammatory cytokines, TNF-α, IL-1β, IL-2, and IL-6, in local blister fluid, circulating plasma, and Cerebral Spinal Fluid (CSF). Pro inflammatory cytokines excite nociceptors and can induce long-term peripheral sensitization. An increase in Calcitonin Gene-related Peptide (CGRP) is also found, so that neuropeptides, substance P and CGRP, antidromically released from sensory terminals in the skin evoke dilatation and protein extravasation in the tissue, a phenomenon known as neurogenic inflammation. Moreover, the production of reactive oxygen species in the affected limb is possibly responsible for the endothelial dysfunction observed in CRPS patients. The impaired endothelial function is a major factor in the pathogenesis of the trophic changes that are found in both superficial and deep tissues [5].
Diagnostic criteria have been approved by the IASP (International Association for the Study of Pain) [6,7], and more recently, a modified version of diagnostic criteria for CPRS, called Budapest Criteria, has been validated by a Harden study [8,9].
Several therapeutic methods for CPRS-I are in use, including the treatment with Bisphosphonates, (BPs) which has gained some success as recent meta-analyses confirmed [10,11]
Therapeutically sympathetic blocks, which inhibit the sympathetic innervation of deep structures and the skin, can be used to alleviate pain [12] even though further confirmations are necessary for a definite judgment [13].
Pharmacological treatment is directed to a particular pathophysiology or current symptoms. Myofascial dysfunction, almost invariably present, with alloying and/or hyperalgesia, requires the use of muscle relaxants, analgesics, and antidepressants. Severe alloying may require a trial of anticonvulsants, desensitisation, or some intervention such as spinal cord stimulation [14].
Material and Methods
Aim of the study
The aim of the studyis to investigate the outcome of an integrated treatment, anestheticblock of sympatheticlumbarchain and neridronateinfusionprotocol, whichhave an effect on both the inflammatory component and the impaired autonomic function [15].
Materials and methods
The treatment consists of a repeated anesthetic block of sympathetic lumbar chain and neridronate infusion protocol. Twelve patients affected by CPRS-I of lower limb have been treated, 4 males and 8 females. Their verge age was 53, 25 ± 10, 3 years and 5 of them had the disease located in the hip, 2 in the knee, 4 in the ankle and 1 in the metatarsal bone. The duration of pain was 68, 58 ± 41, 82 days. In order to be enrolled, patients had to fulfill Budapest’s diagnostic criteria for CPRS-I [9], with a further confirmation of the diagnosis deriving from MRI, which detects edema of trabecular bone, and/or triphasic bone scintigraphy.
VAS scale hasbee nevaluated for each patient before each session to quantify the pain and the subjective improvement hasbeen measuredusing Subjective Rating Scale of functionrecovery. The above-mentioned scale consists of 5 levels, whichcorrespond to the following points: 0= no recovery, 1= slight recovery, 2= moderate recovery, 4= excellent recovery.
Microcirculation hasbeen analysed with laser-doppler fluometry (LDF; Periflux, PF4000, Perimed AB) bothbefore the beginning of the therapy and at the end of the treatment, placing the probe on the affected area.
Periflux system, by exploiting the Doppler effect, allows a real time continuous measuring of cutaneous microcirculation. Detected parameters are Perfusion Unity (PU), deriving from the product of velocity (v) and concentration of moving blood cells (CMBC), and the analysis of vasomotion, which represents the rhythmic oscillation of vascular tone, due to contraction and relaxation of local smooth musculature. This activity reduces with the restriction of the diameter of vessels. Cutaneous microcirculation is regulated by a dynamic interaction of different factors [16], such as sympathetic system and endotelium-mediated factor. In response to physical stimuli, endothelium releases not only endothelium-derived vasodilators, such as NO, bradykinin, prostacyclin and Endothelium-Derived Hyperpolarizing Factor (EDHF), but also vasoconstrictors, such as ET-1 and angiotensin II [17]. The influence of those factors on the genesis of vasomotion is identified by a characteristic range of frequencies represented in the oscillations of the curve [18]. Therefore, the spectral analysis of detected oscillations allows us to identify the role of each regulation factor in the genesis vasomotion. In this study, we have considered different ranges of frequency, which include the one related to myogenic activity from 0,052 to 0, 15 Hz, the one due to sympathetic activity between 0,021 and 0,052 Hz, and the last one, covering slow oscillations from 0.0095 to 0.021 Hz, shows the endothelium dependent factor [18].
The exact distribution of registered frequencies has been analysed by the Perisoft for Windows system.
Protocol of neridronate infusion
Neridronate, an aminobisphosphonate, is a potent inhibitor of bone resorption and bone remodeling. It is approved and marketed in Italy for bone diseases, e. g. Paget’s disease of bone, osteogenesisim perfecta, and algodystrophy (CRPS type I) [19]. The therapeutic protocol for the treatment of algodystrophy consists of the infusion of 100 mg of neridronate four times in ten days. The infusion has to be made using 250 mL of physiological saline solution in 2-3 hours.
Sympathetic lumbar ganglion block
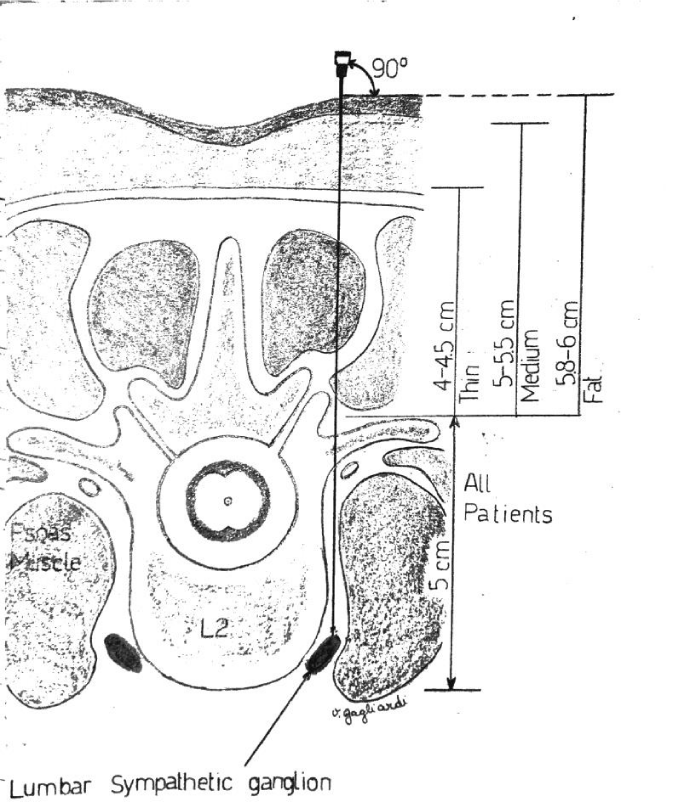
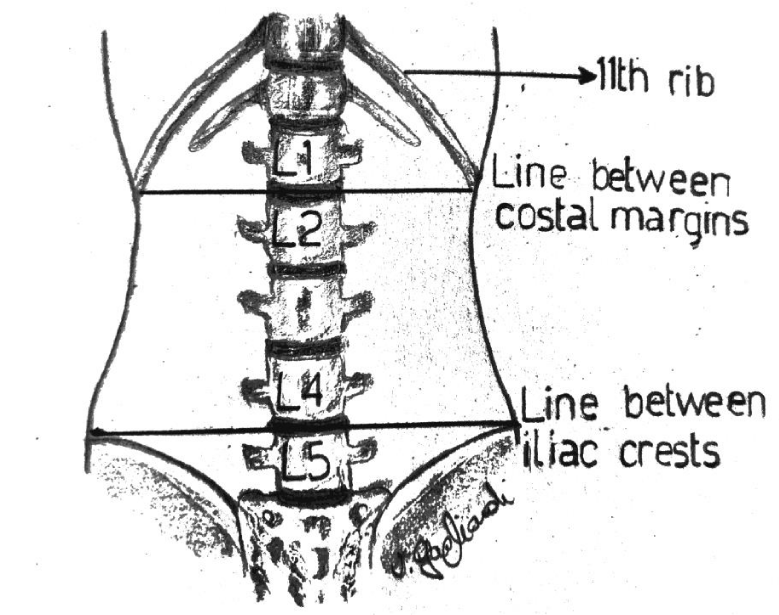
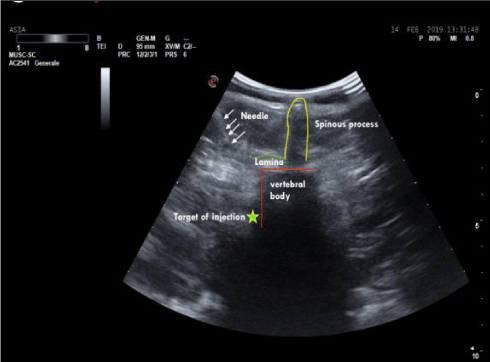
In the lumbar segment, sympathetic ganglia are located on the anterolateral surface of vertebral bodies and on the medial border of psoas muscle. Anatomical relationships between the spinous and the transverse process, and between the latter and the ganglion are usually constant. The distance between the transverse process and the ganglion is 4-5 cm, considering that the anteroposterior diameter of the vertebral body has a variation of about 0, 6 cm due to the patient’s height. The distance in depht from the spinous to the transverse process generally measures 3,5 cm (Figure 1). Consequently, in this context, the higher variability derives from the quantity of the muscular mass and the adipose tissue. The greatest component of the sympathetic innervation of the lower limb derives from L2 ganglion, a minor component is given by L3 and L4. In this study, a single injection on the ganglion situated at L2 level has been used. The patient is carefully positionated in lateral decubitus, with the thighs bent on the abdomen and the head bent on the thorax. In order to exactly define the position of L2 body, the following procedure has been carried out: Firstly, a line passing bilaterally through the costal border has been traced, intersecting the spinous process of L2 or the interspace between L1 and L2. Secondly, another line has been traced joining the two iliac crests. It intersects L4 spinous process or the interspace between L4 and L5 (Figure 2). The exact point of the puncture is situated 4-5 cm laterally of the spinous process of L2 (20). A needle 22-gauge (Spinal, PIC) has been employed, doing the first insertion at 45° on skin until the contact with the transverse process. After having reached that distance, the needle is withdrawn and reinserted with an inclination of 90° on the skin, 4 cm deeper than the distance previously identified in relation to the position of the transverse process. The procedure of puncture and the assessment of the exact placement of the needle have been monitored under ultrasound guide (Figure 3). After repeated aspirations which evaluate the possible presence of blood or liquor, levobupivacaina 0, 5% has been injected slowly; the administered dosage was 1 mg/ kg.
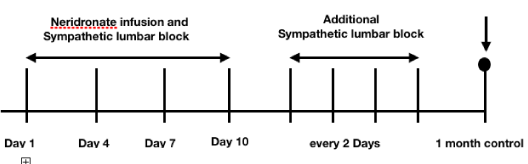
The time table (Figure 4) shows the pattern of the treatment: in the days 1-4 - 7-10 patients underwent the neridronate infusion and, at the same time, the lumbar sympathetic nerve block. At the end of the 10 days, whether the VAS value was higher than 40, the treatment would be continued with lumbar sympathetic nerve block every 2 days until the VAS value was lower than 40. The patients have been evaluated 1 month after the end of the treatment for the follow-up.
Results and Discussion
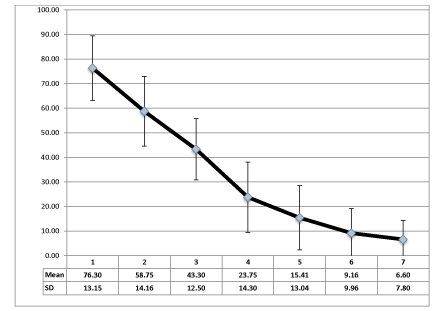
All patients have expressed a rapid relief of pain and its progressive reduction has become superior than the 50% of the initial value at the 4° treatment (Graph 1). Our results have been analysed with the Paired Sample T-test, and they are significative (p < 0.05).
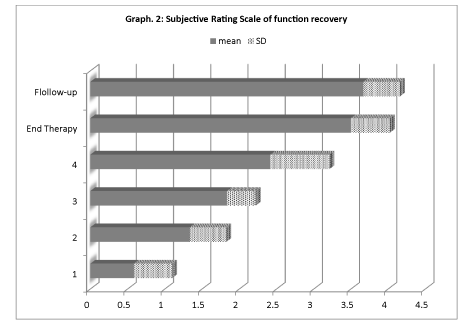
Moreover, the significant improvement in the motor function which has occurred after the third treatment, has shown to be mantained at the 1 month control (Graph 2). So, to maximize this effect, each patient has undergone 7,6 ± 2.06 sympathetic nerve blocks.
Laser dopplerfluometry
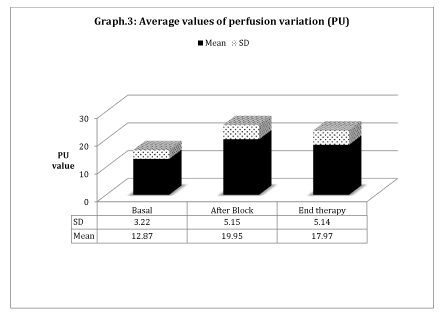
Data deriving from the microcirculation study of the affected area highlight a vasodilatation reaction confirmed by the clinical observation: the color of the skin changes and a warm sensation is reported, lasting for some hours after the nerve block. Average values of Perfusion Variation (PU) analysis show an increase in perfusion in the aforementioned zone, which reaches the maximum after 30 minutes from the block, but persisting until the end of the therapy (Graph 3).
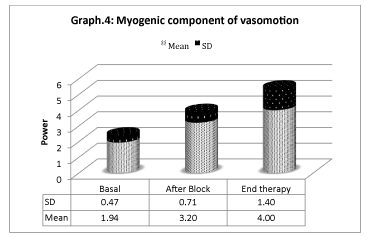
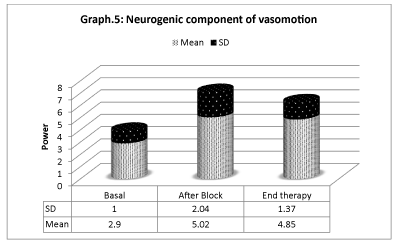
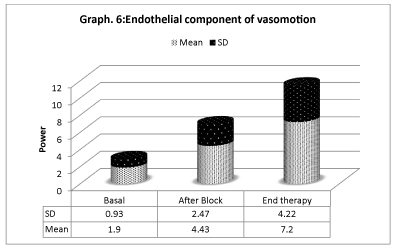
Vasomotion analisys shows that microcirculation variations (Graphs 4-6) are mostly associated with slow endothelial oscillators, but are also correlated with neurogenic and myogenic components, indicating a global cooperation of multiple factors during vasomotion with higher/lower components. Considering the neurogenic component (0.021- to 0.052 Hz), a significative variation is detected especially after the sympathetic block, but it has also persisted for the period after. The same variation is registered on the myogenic component. In spite of that, the most significative component derives from the lower frequencies representing the endothelial component, showing that the role of metabolic regulation is considerable.
Discussion
In all patients, the associated treatment of sympathetic nerve block and neridronate infusion has been demonstrated to be effective in the improvement of symptoms without significant side effects. The detected rapid control of pain is fundamental for the functional recover of the affected limb. The local anesthetic injection on lumbar sympathetic ganglia causes a vasodilatation response. On account of this, patients report an immediate warm sensation and their skin becomes epidemic. Cutaneous flow expressed as PU, at the end of the treatment increases by 80% of the basal value.
This rapid first response is due to the interruption of sympathetic vasoconstriction consequent to the nerve block. Nevertheless, spectral analysis of oscillation frequencies of vasomotor indicates that other mechanisms may be implied. On the one side, a power increase in the component of frequencies related to the sympathetic system is registered, indicating that the neurogenic regulation of microcirculation is modified. On the other side, a significative variation of both the components concerning the endothelial function and the one due to the smooth musculature activity occurs, showing a restoration of the flow regulation.
These results lead to the hypothesis that, at least in this phase, there is an important contribution of the autonomic system in the persistance of symptoms, but it confirms the importance of the variation of the endothelial function.
Therefore, the sympathetic nerve block with the local anesthetic produces a notable sudden analgesia. Futher more, treatment with biphosphonates reduces pain in patients with Complex Regional Pain Syndrome type I, but the antiosteoclastic action of bisphosphonate cannot explain the potentially antalgic effect [11].
Both the pain and the microcirculation impairment in CPRS-I could well be the consequence fan inflammatory process, in fact researches have highlighted a pivotal role for inflammation in the pathophysiology of CRPS. In this framework, inflammation can be the cause of the endothelial dysfunction [21].
Our results could be explained by a sinergicant inflammatory effect of the two administered treatments. The ant inflammatory mechanism of biphosphonates consists of the reduction in the release of cytokines, including TNF α, IL-1, IL-6 and Nerve Growth Factor (NGF) [16]. They facilitate the liberation of neuropeptides, substance P and calcitonin gene-related peptide, which produces vasodilation, increased microvascular permeability, protein extravasations and oedema, causing a process of neurogenic neuroinflammation. The resulting impaired microcirculation probably maintains and worsens the disease, generating the final picture of CRPS-I, characterised by metabolic tissue acidosis.
In this framework, the sympathetic nervous system probably contributes by interacting with the above-described mechanisms, producing vasomotor disturbances [10]. The sympathetic nervous system and inflammation interact: norepinephrine influences the immune system and the production of cytokines. There is substantial evidence that this interaction contributes to the pathophysiology and clinical presentation of CRPS, even though this interaction is not straightforward [22].
Different mechanisms have been proposed: an aberrant expression of α1-adrenoreceptors on immune cells contributes to inflammation and pain during CPRS. More recently, autoimmune contributions have been suggested by the discovery of self-directed pain-promoting IgG and IgM antibodies in CRPS patients and model animals. Both the autoimmune and the auto inflammatory components of CRPS appear to be regulated by neuropeptide-containing peripheral nerve fibers and the sympathetic nervous system [23].
Sympathetic signalling blockers may reduce both auto inflammatory and autoimmune responses.
Conclusion
In conclusion, the aforementioned synergy could be the main mechanism causing the therapeutic effect observed in our case study. The reduction of hypoxic injury in deep tissues and the restoration of homeostatic nor epinephrine signaling lead to a rapid clinical improvement in CPRS. Therefore, a sinergicant inflammatory action of neridronate infusion and repeated sympathetic lumbar chain block can be hypothesized, with a consequent restoration of microcirculatory function. However, further studies which include a higher number of patients are necessary to better define this hypothesis and the underlying mechanisms.
Acknowledgement
We want to thank Dott. Baldassare Licata for technical consultancy for the execution of the sympathetic ganglion block.
- de Mos M, Huygen FJ, Dieleman JP, Koopman JS, Stricker BH, Sturkenboom MC. Complex regional pain syndrome (crps). Pain. 2008; 139: 458-466. http://bit.ly/2NneFEj
- Bruehl S. Modifying diagnostic criteria for complex regional pain syndrome. Pain. 2010; 150: 217-218. http://bit.ly/2MYYpKA
- Stanton-Hicks M. Complex regional pain syndrome. In fundamentals of pain medicine. Jianguo Cheng Richard W. Rosenquist Editors. 2018; 211-220.
- de Mos M, Sturkenboom MC, Huygen FJ. Current understandings on complex regional pain syndrome. Pain Practice. 2009; 9: 86-89. http://bit.ly/2pofHb3
- Kortekaas MC, Niehof SP, Stolker RJ, Huygen FJ Huygen F. Pathophysiological mechanisms involved in vasomotor disturbances in complex regional pain syndrome and implications for therapy: a review. Pain practice. 2016; 167: 905-914. http://bit.ly/2PxdnZN
- Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010; 113: 713-725. http://bit.ly/2PuKbm l
- Bruehl S. Modifying diagnostic criteria for complex regional pain syndrome. Pain. 2010; 150: 217-218. http://bit.ly/2MYYpKA
- Harden RN, Bruehl S, Perez RS, Birklein F, Marinus J, Maihofner C, et al. Validation of proposed diagnostic criteria (the “budapest criteria”) for complex regional pain syndrome. Pain. 2010; 150: 268-274. http://bit.ly/2NmW5Ml
- Harden RN. Objectification of the diagnostic criteria for crps. Pain med. 2010; 11: 1212-1215. http://bit.ly/2WtIPdc
- Giusti A, Bianchi G. Treatment of complex regional pain syndrome type I with bisphosphonates. RMD Open. 2015. http://bit.ly/2NnIgNF
- Chevreaua M, Romanda X, Gaudina P, Juvina R, Bailleta A. Bisphosphonates for treatment of complex regional pain syndrome type 1: a systematic literature review and meta-analysis of randomized controlled trials versus placebo. Joint Bone Spine. 2017; 84: 393-399. http://bit.ly/2Ps8SQe
- Gungor S, Aiyer R, Baykoca B. Sympathetic blocks for the treatment of complex regional pain syndrome: a case series. Medicine. 2018; 97: e0705. http://bit.ly/31ZOMPU
- Kortüm FC, Brascher AK, Schmitz-Buchholz D, Feldmann RE Jr, Benrath J. Sympathetic blocks for complex regional pain syndrome: a survey of pain physicians. Regional Anesthesia & Pain Medicine. 2019; 44: 736-741. http://bit.ly/2JyGp7T
- Bussa M, Guttilla D, Lucia M, Mascaro A, Rinaldi S. Complex regional pain syndrome type I: a comprehensive review. Acta Anaesthesiol Scand. 2015; 59: 685-697. http://bit.ly/2ou29KJ
- Russo M, Georgiusb P, Santarelli DM. A new hypothesis for the pathophysiology of complex regional pain syndrome. Med Hypotheses. 2018; 119: 41-53. http://bit.ly/331Ji8E
- Kingery WS. Role of neuropeptide, cytokine, and growth factor signaling in complex regional pain syndrome. Pain Med. 2010; 11: 1239-1250. http://bit.ly/2NB83lV
- Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003; 3: 79-89. http://bit.ly/2MXrUML
- Hodges GJ, Del Pozzi AT. Noninvasive examination of endothelial, sympathetic, and myogenic contributions to regional differences in the human. Microvasc Res. 2014; 93: 87-91. http://bit.ly/3494GJe
- Varenna M, Adami S, Rossini M, Gatti D, Idolazzi L, Zucchi F, et al. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology. 2013; 52: 534-542. http://bit.ly/2NmXlix
- Moore D: regional Block, Springfield, Charles C. Thomas. 1981; 211-218.
- Wang L, Guo TZ, Hou S, Wei T, Li WW, Shi X. Bisphosphonates inhibit pain, bone loss, and inflammation in a rat tibia fracture model of complex regional pain syndrome. Anesth Analg. 2016; 123: 1033-1045. http://bit.ly/36fnKHL
- Schlereth T, Drummond P, Birklein F. Inflammation in CRPS: role of the sympathetic supply. Autonomic Neuroscience: Basic and Clinical. 2014; 182: 102-107. http://bit.ly/2PufAW0
- Clark JD, Tawfik VL, Tajerian M, Kingery WS. Autoinflammatory and autoimmune contributions to complex regional pain syndrome. Mol Pain. 2018; 14: 1-13. http://bit.ly/2MZ1pXx

Sign up for Article Alerts