Determination of pKa from Solubility Measurements in Simple Cases when No Additional Equilibria are Present?
Julia Martin1*, Sarra El Moussaoui2 and Agustin G. Asuero2
1Department of Analytical Chemistry, Escuela Politécnica Superior, University of Seville, 41011-Seville, Spain
2Department of Analytical Chemistry, Faculty of Pharmacy, University of Seville, 41012-Seville, Spain
*Address for Correspondence: Julia Martin, Department of Analytical Chemistry, Escuela Politecnica Superior, University of Seville. C/ Virgen de Africa, 7, E-41011 Seville, Spain, Tel: +349-545-562-50; E-mail: [email protected]
Submitted: 18 July 2018; Approved: 31 August 2018; Published: 03 September 2018
Citation this article: Martin J, Moussaoui SE, Asuero AG. Determination of pKa from Solubility Measurements in Simple Cases when No Additional Equilibria are Present. Int J Pharma Anal Acta. 2018;2(1): 015-021.
Copyright: © 2018 Martin J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: pKa; Solubility measurements; Calcein blue; Butaperazine; Sulfadiazine; Tyrosine; 8-Hydroxiquinoleine; Niflumic acid
Download Fulltext PDF
A poor solubility in water limits in a drastic way the efficacy of a drug, because the absorption phenomenon requires the drugs be in dissolution. The therapeutic activity of a drug is depending of its acid-base dissociation constant (pKa) and solubility, the knowledge of pKa values being thus of great worth. The solubility method can be very useful in spite of their limitations if an appropriate method is available to carry out the solubility measurements of scarce solubility compounds. Some examples taken from the bibliography whose behaviour is well adapted to conventional acid-base dissociation equilibria without further complications are selected for study: Calcein blue, butaperazine, sulfadiazine, tyrosine, 8-hydroxiquinoleine and niflumic acid. The pKa values have been recalculated applying the single least squares method and the classic monoprotic acid bilogarithmic model. A slope-intercept procedure is also applied to get the evaluation of acidity constants of overlapping equilibria (pKa2 and pKa3 of tyrosine). Results obtained in all cases are compared with literature data.
Introduction
The efficacy of a drug may be drastically limited due to its poor solubility in aqueous medium, since only dissolved substances can be absorbed [1]. In addition, poor solubility can lead to side effects. The therapeutic activity of a drug is depending of its acid-base dissociation (pKa) and solubility [2]. The knowledge of pKa values [3] is of great worth in order to solve some galenical questions, solubility being in addition a parameter important to devise the elaboration of formulations. Many biological compounds have also nearby acidity constants. Its absorption, subsequent transport and effect on the living organism are affected by the concentration ratio of protonated to non-protonated forms [4,5], so knowledge of the acidity constants is of great worth.
The most common and simple acid-base potentiometric titration method is not generally applicable in the case of sparingly soluble compounds [6]. There are also compounds whose conjugated acid-base forms have similar absorption spectra. The solubility method, although laborious, shows its usefulness in these cases, when a proper analytical method allows performing the measurements of solubility. Some examples of compounds (calcein blue, butaperazine, sulfadiazine, tyrosine, 8-hydroquinoline and niflumic acid), whose behaviour is well adapted to conventional acid-base dissociation equilibria without further complications, have been selected from the bibliography for study.
Single least squares method and the classic monoprotic bilogarithmic method have been applied to re-evaluate the pKa values of the compounds subject of study. For very close pKa values (pKa2 and pKa3 of tyrosine) a slope-intercept procedure is applied. Residual analysis, error analysis and t-testing slope (bilogarithmic method) is carried out in both kind situations. Results obtained in all cases are compared with literature data.
Determination of Acidity Constants from Solubility Measurements
The method of solubility measurements for the evaluation of pKa is quite laborious [1,5-12], but useful when:
• The substance is too insoluble in water to apply the potentiometric or conductometric methods
• The UV-visible spectra of molecular and ionic forms are either very similar or lack it.
The fact that the spectra are identical does not prevent the analysis of saturated solutions, which can be determined gravimetrically (after precipitation with a reagent) or spectrophotometrically (after the addition of a chromogenic reagent). The sensitivity of the method can be greatly increased by labelling the substance with a radioactive isotope.
A sparingly soluble acid or base is partitioned between the solid and a saturated solution. The concentration of a neutral acid, HR, (s0)
(1a,b)
or a neutral base, B (s0)
(2a,b)
will be constant in a saturated solution, but the total solubility will vary with pH. Parentheses in Eqns. (1) and (2) denote activities and brackets concentrations. It is necessary to carry out the determinations ionic and constant temperature. Then a mixed or Bronsted acidity constant is obtained.
Solubility is measured over a wide pH range by a standard analytical method. The value of the intrinsic solubility, s0, obtained by extrapolation (Table 1) of solubility at low pH values (HR) or high pH values (B). The logarithmic equations to be applied in the evaluation of acidity constants, obtained by rearranging Eqns. (1a) and (1b) in the case of the neutral acid HR, and (2a) and (2b) in the case of the B base are shown in table 1. In practice, an excess of problem is stirred in the thermostated bath with buffer solutions under inert atmosphere until a fixed concentration is found by analysis. The dissolved solute is separated by centrifugation and the pH is measured immediately. Albert and Serjeant [6] gives useful recommendations.
A representation of the logarithmic of the quotients between solubility and intrinsic solubility minus one against pH gives a straight line of slope unit (acid) or minus slope unity (base) that intersect the x-axis at the pKa values
(3)
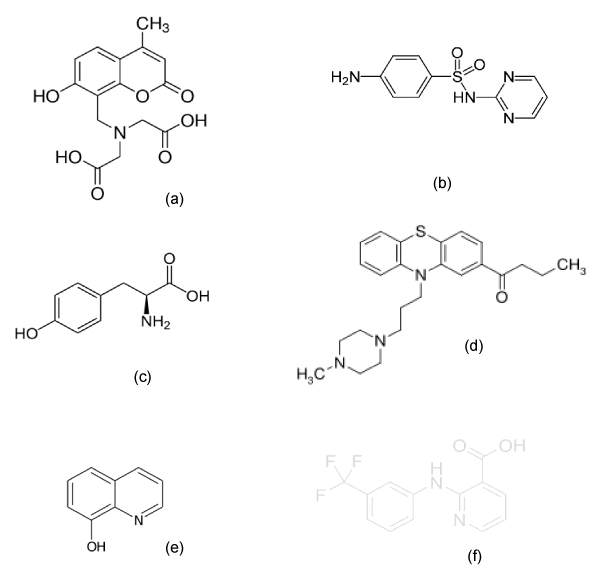
Deviations of the slope from its nominal value could indicate a problem with the model, for example, additional equilibria. Next we are going to calculate the values of acidity constants of the chemical systems indicated above described in the bibliography. The solubility data as a function of the pH of these systems are shown in table 2 [13-18]. In figure 1 their structural formulas are shown.
Calcein Blue
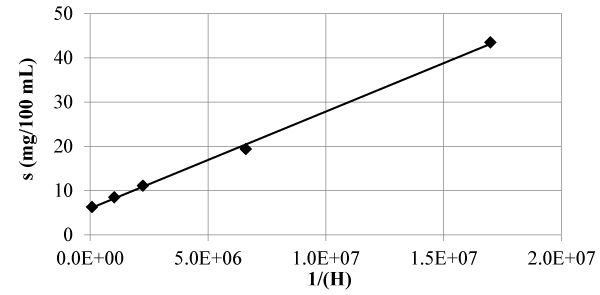
Calcein blue is a fluorescent metallic indicator [13,19], which is obtained by condensation between 4-methyl-umbelliferone, formaldehyde and iminodiacetic acid. The pKa1 of calcein blue is very low, so its intrinsic solubility is not susceptible to direct measurement, being obtained by extrapolation of the line of solubility against the inverse of the activity of the protons, obtaining a value equal to 1.00 mg/ 100 mL.
Sulfadiazine
Sulfadiazine is a sulfonamide type antibiotic. It is an amphoteric compound of the HR (=s0) type [14,20], which is protonated in an acidic medium and deprotonates in basic medium, forming charged species in both cases, H2R+ and R-, respectively, with the consequent increase in solubility
(4a,b,c)
The intrinsic solubility is close to the minimum solubility obtained in the central part of the curve, since the pKa’s are not very close. The plot of the solubility against the activity of the protons in the alkaline side (Figure 2), or against the inverse of the activity of the protons in the acid part, leads to a mean value of the intrinsic solubility of 6.00 mg/ 100 mL.
Tyrosine
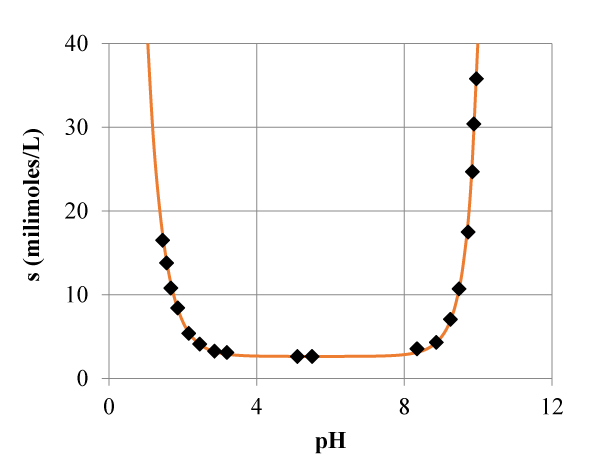
Tyrosine is one of the twenty amino acids that make up proteins. It is classified as nonessential in mammals, since its synthesis is produced from the hydroxylation of another essential amino acid: phenylalanine. It is an H2R-type substance, amphoteric, which is protonated in an acid medium, and in a basic medium suffers a double deprotonation, so it has three constants of acidity
(5a,b,c)
Since this phenomena occur independently, the intrinsic solubility, s0=[H2R], can be considered as the minimum (2.62 millimoles/L) in the graph (Figure 3) of solubility against pH.
In the basic medium, two simultaneous deprotonations occur and then taking into account Eqns. (4a,b,c) the solubility is given by
(6)
which on rearranging we finally get
(7)
A representation of the member on the left of the Eqn. (7) versus the inverse of the activity of the protons leads to a straight line of the form, y = a0 +a0 x, from whose intercept and slope the values of acid constants sought are calculated as
(8)
Butaperazine
Butaperazine is an antipsychotic used in the treatment of schizophrenia and chronic brain syndrome belonging to the phenothiazine class. It is a diacid base (B) with well-separated acidity constants [21]; solubility data in table 2 are given only for the basic side. The intrinsic solubility calculated by plotting solubility against proton activity is equal to 2.067 mg/ 100 mL.
8-Hydroxyquinoline
The 8-hydroxyquinoline or oxine is a monoprotic (HR) amphoteric bidendate chelating agent. It has been used for a long time in metal analysis due to its chelating power. Its complexes and the reagent itself have antiseptic and disinfectant properties [22].
The logarithmic equations compiled in table 1 may be first applied first, taking in first instance the minimum solubility value as the intrinsic solubility.
From Eqn. (4a,b,c) we get
(9a,b,c)
With the values of acidity constants evaluated a better value of the intrinsic solubility may be calculated. By substituting Eqn. (9c) in Eqn. (9a), we get the si value and on rearrangement we have
(10)
Once a better value of the intrinsic solubility is known, s0= 0.549 g/L after a single coarse cycle (the acidity constants are well separated) the logarithmic equations (Table 1) are then applied.
10. Niflumic Acid
It is a non-steroidal anti-inflammatory drug used to relieve joint and muscle pain. It is an amphoteric substance of the HR type.
Error Analysis
The law of error random propagation [23], applied to a function R=f(a0,a1) gives
(11)
sa02, sa12 and cov(a0,a1) are the variance of the intercept, the variance of slope and the covariance between the slope and intercept, respectively, obtained by means of the least squares method.
In the case of the logarithmic method from Eqn. (3) by simple algebra we get finally
(12)
In the case of tyrosine, from Eqns. (8a,b) the following expressions are derived for the standard deviations of pKa2 and pKa3
(13)
(14)
A detailed treatment of the error analysis applied (derivation of the formulas involved) to the evaluation of acidity constants may be seen [24] in a recent paper. LINEST function in Excel [25,26] gives all necessary parameters with the exception of the cov(a0,a1)
LINEST gives the standard deviation of the regression line, sy/x. The sum of squares about the mean of the x values, SXX, is directly calculated in Excel with the DEVSQ function (SOMME.CARRES.ECARTS in French and SUMQUADABW in German [25], and DEVSQ also in Spanish).
Evaluation of Mixed (Bronsted) Acidity Constants
The results obtained by applying the bilogarithmic method, usually known as Krebs and Spealman method [6,14] (Table 1) to the experimental data compiled in table 2 with the aid of the least squares procedure are shown in table 3. Values of estimated mixed (Bronsted) pKaB constants, from now on called pKa through the paper, are reported in this table with three decimal digits in all cases even if they are not significant. Ionic strength is equal to 0.1 for calcein blue, sulfadiazine, and 8-hydroxiquinoleine systems. It is assumed to be constants for the niflumic acid [16] and butaperazine [18] (as no information is given for them). However, data for tyrosine were obtained [15] at varying ionic strength. In the case of the calculation of overlapping pKa2 and pKa3 of tyrosine the slope intercept procedure corresponding to Eqn. (7) is applied.
The number of points selected in each case, the relevant values of the slope and intercept, and the corresponding standard errors associated, together with the R2 value, are included in the table 3. Values very close to the intrinsic solubility were discarded in the calculations. The applicability of the simple bilogarithmic model (Table 1) may be checked by means of the Student t-test. The difference between the experimental slope and the unity (absolute value) theoretical slope value is significant if the value of t experimental
(15)
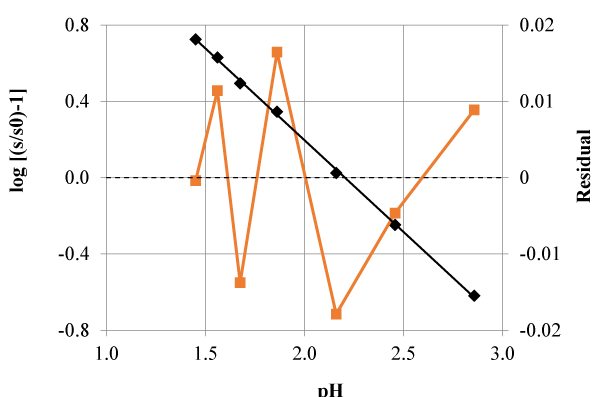
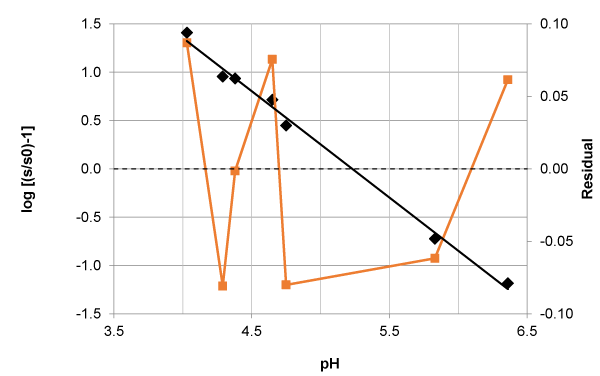
is higher than the value tabulated for t for k-2 degrees of freedom and the 95% confidence, t (k-2, 0.05). A search to Table 3 shows that significant differences are obtained with tyrosine (pKa1) and 8-hydroxiquinoleine (pKa1).
In the case of the pKa1 of the tyrosine system ionic strength is assumed to be constant. In spite of this approximation, as results are very precise, the small standard deviation of the slope is the reason why calculated (experimental) t value exceeds the tabulated one. The slope is about a four per cent away from the unity. In the case of the pKa1 of 8-hydroxiquinoleine system the calculated value of t is only slightly superior to the tabulated one. However the deviation from the theoretical slope is greater in this case, of the order of a ten per cent. In both cases residual analysis (Figures 4 and 5) have a random pattern suggesting a valid model from (only) this point of view.
Weighting
The weighting factors wi for the weighted least squares [27,28] are given (assuming that pH is free from error and random errors are mainly concentrated in solubility measurements) by
(16)
as s is converted into the logarithmic term in Eq.0 (1b, 2b). The weights calculated from Eqn. (10) may be normalized [25,26]. The application of the weighted linear regression instead of the single linear regression leads to similar results, not being applied, as it is in this case an unnecessary complication.
Final Considerations: No Models Perfect
The therapeutic activity of the drugs (the absorption, further transport and effect of compounds having biological interest) is related to their free concentration available in the plasma, which depends, among other factors, on the solubility and ionization of the substance. The bilogarithmic (and the slope-intercept) method for the evaluation of pKa from solubility measurements works well with systems whose behaviour is ideal. We have occasion to check the single model in this paper, with some acid, base and amphoteric compounds.
This means that no additional equilibria appears superimposed to the conventional acid-base equilibria involved. However, the real world is usually more complex that simple models assume. Box [29] stated, “All models are wrong”. As a matter of fact [30] there are not perfect models, but models that are more adequate than others. Any additional complication possible (change in ionic strength, subtle effects of buffer components, drug precipitation, aggregate formation, etc) is rigorously analysed by the Dr. Alex Avdeef (please give a search to Alex Avdeef Google Scholar Citations) with the aid of his p-DISOL-X program [31] (which uses Stockes-Robinson hydration model for the adjustment of ionic strength). A cationic aggregate (dimer) model e.g., may be included (private communication) in the study of the 8-hydroxiquinoleine system.
- Avdeef A. pKa determination. In absorption and drug development: solubility, permeability, and charge state. 2nd edition. New York: Wiley; 2012. 1-129. https://goo.gl/tvsZEC
- Asuero AG. A bilogarithmic method for the evaluation of acidity constants of amphoteric substances from solubility measurements. Int J Pharm. 1989; 52: 129-137. https://goo.gl/hStJ4K
- Newton WD, Ratanamaneichatara S, Murray JW. Dissociation, solubility and lipophilicity of azathioprine. Int J Pharm. 1982; 11: 209-213. https://goo.gl/3B6NQ9
- Avdeef A. Solubility of sparingly-soluble ionizable drugs. Adv Drug Deliv Rev. 2007; 59: 568-590. https://goo.gl/Rj2A1s
- Brittain GH. Ionic equilibria and the pH dependence of solubility. New York: Springer; 2007. https://goo.gl/oUhb2c
- Albert A, Serjeant EP. The determination of ionization constants: a laboratory manual. 3rd edition. London: Chapman and Hall; 1984. https://goo.gl/K7xU3N
- Avdeef A. Absorption and drug development: solubility, permeability, and charge state. New York: Wiley; 2003. https://goo.gl/pQ7x1N
- Baka E. Development and examination of solubility measurement methods for drug solubility determination. Budapest: Semmelweis University; 2010. https://goo.gl/Fp8a6B
- Bruno B. Development of new experimental tools for fast. Determination of solubility and lipophilicity. Geneva: Université de Genève; 2008. https://goo.gl/dzRAow
- Butler JN, Cogley DR. Ionic equilibrium. Solubility and pH calculations. New York: Wiley; 1998. https://goo.gl/oXbTHp
- Cookson RF. The determination of acidity constants. Chem Rev. 1974; 74: 5-28. https://goo.gl/deVZzo
- Ramette RW. Chemical equilibrium and analysis. Addison-Wesley; 1981. https://goo.gl/c9ukUa
- Huitink GM, Diehl H. Methyl calcein blue and other analogues of Calcein Blue. Talanta 1974; 21: 1193-1202. https://goo.gl/ZhsLBC
- Krebs HA, Speakman JC. Dissociation constant, solubility, and the pH value of the solvent. J Chem Soc. 1945; 1: 593-595. https://goo.gl/e5Pdv2
- Hitchcock DI. The solubility of tyrosine in acid and in alkali. J Gen Physiol. 1924; 6: 747-757. https://goo.gl/myWjb1
- Zimmermann I. Determination of overlapping pK values from solubility data. Int J Pharm. 1986; 31: 69-74. https://goo.gl/SuU791
- Irving H, Ewart JAD, Wilson JT. The dissociation constants of 8-hydroxy quinoline. J Chem Soc. 1949; 1: 2672-2674. https://goo.gl/jCf5Wj
- Bres J, Bressolle F, Huguet MT. Importance of ionic dissociation of drugs in pharmacokinetics. Methods of determining their pKa. Trav Soc Pharm Montpellier. 1976; 36: 331-364.
- Huitink GM, Poe DP, Diehl H. On the properties of calcein blue. Talanta. 1974; 21: 1221-1229. https://goo.gl/29A7Lv
- Zimmermann I. Determination of pKa values from solubility data. Int J Pharm. 1982; 13: 57-65. https://goo.gl/LFhD1J
- Kala H, Wendorff D, Moldenhauer H. Investigations on the solubility and distribution behaviour of butaperazindibimaleinate. Pharmacy. 1980; 34: 306-310.
- Martin J, Ropero Alés M, Asuero AG. An overview on ligands of therapeutically interest. Pharm Pharmacol Int J. 2018; 6: 198-214. https://goo.gl/9cEGUK
- Asuero AG, Gonzalez G, de Pablos F, Ariza JL. Determination of the optimum working range in spectrophotometric procedures. Talanta. 1988; 35: 531-537. https:// goo.gl/msRnzJ
- Martin J, Hidalgo-Soria A, Asuero AG. Spectrophotometric evaluation of acidity constants: can a diprotic acid be treated as a monoprotic one. J Lab Chem Educ. 2018; 6: 107-117. https://goo.gl/pjA24L
- Billo EJ. Excel for chemists, a comprehensive guide. 3th edition. New York: Wiley; 2011. https://goo.gl/Rromja
- Liengme BV. A guide to microsoft excel 2013 for scientists and engineers. Amsterdam: Elsevier; 2015. https://goo.gl/8Rfzdk
- Asuero AG, González AG. Some observations on fitting a straight line to data. Microchem J. 1989; 40: 216-225. https://goo.gl/eLaFN5
- Asuero AG, Gonzalez A. Fitting straight lines with replicated observations by linear regression. III. Weigthing data. Crit Rev Anal Chem. 2007; 37: 143-172. https://goo.gl/LcP9uK
- Box GEP, Hunter JS, Hunter WG. Statistics for experimenters. 2nd edition. New York: Wiley; 2005. https://goo.gl/ewkGmu
- Martin J, Romero-Gracia A, Asuero AG. Fitting non-linear calibration curves: no models perfect. J Anal Sci Meth Inst. 2017; 7: 1-17. https://goo.gl/HxGV5L
- In-ADME Research. 2011. https://goo.gl/KmRZdT

Sign up for Article Alerts