Effect of Clofibrate, A PPAR-Α Receptors Agonist, On Behavioral Despair Associated With Exposure to Forced Swim in Rats
Nahid Fakhraei1#, Raghda Javedan2,3#, Vahid Nikoui3, Azam Bakhtiarian3 and Seyed Said Pournaghash Tehrani2*
1Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
2Department of Psychology, School of Psychology, Tehran University, Tehran, Iran
3Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
#These two authors contributed equally in this study
*Address for Correspondence: Seyed Said Pournaghash Tehrani, Department of Psychology, School of Psychology, University of Tehran, Jalal Al Ahmad-Kuye Nasr, P.O. Box 141556456, Tehran, Iran, Tel: +989-374-264-392; E-mail: spnaghash@ut.ac.ir / n.fakhraei@gmail.com
Submitted: 20 November 2017 2017; Approved: 29 December 2017; Published: 30 December 2017
Citation this article: Fakhraei N, Javedan R, Nikoui V, Bakhtiarian A, Pournaghash Tehrani SS. Effect of Clofibrate, A PPAR-Α Receptors Agonist, On Behavioral Despair Associated With Exposure to Forced Swim in Rats. Adv J Toxicol Curr Res. 2017;1(2): 107-115.
Copyright: © 2017Pournaghash Tehrani SS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords : Behavioral Despair; Clofibrate; PPAR-Α Receptor; Forced-Swimming Test; Open Field Test; Rat
Download Fulltext PDF
Background: The potential effect of clobibrate, a Peroxisome Proliferator-Activated Receptor alpha (PPAR-α ) agonist, on behavioral despair associated with acute exposure to Forced Swim Test (FST) was studied in male rats and further, a possible involvement of PPAR-α receptors mediating this effect was suggested.
Methods: There were two swim sessions. The first was a 15 min pre-test and 24 hrs. Later a second 5 min swim test. The 5 min swim test was used for scoring the passive behavior, immobility, and active behaviors, swimming, climbing and diving. Locomotor activity was also evaluated using Open Field Test (OFT). The drugs were administered three times at 2, 19, and 24 hrs. Subsequent to the initial 15 min pre-test, prior to the 5 min test. Clofibrate was received orally (300 mg/kg, p.o.), Desipramine (DMI), a selective Serotonin-Norepinephrine Reuptake Inhibitor (SNRI) (10 mg/kg) and Fluoxetine (FLX), a Selective Serotonin Reuptake Inhibitor (SSRI) (10 mg/kg) were administered intraperitonealy (i.p.).
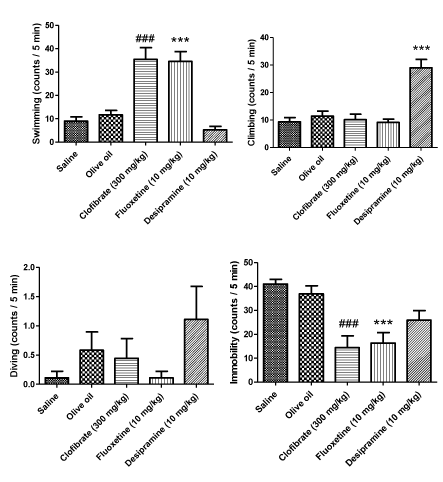
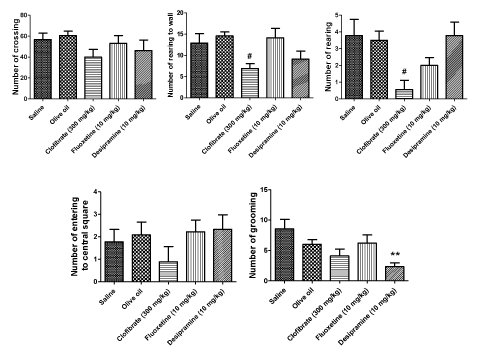
Results: Subchronic clofibrate administration (300 mg/kg, p.o.) attenuated behavioral despair determined by decrease in immobility and increase in the active behavior swimming comparable with FLX (10 mg/kg, i.p.). Clofibrate also markedly reduced the number of rearing and rearing to the wall. That are mentioned as depression like behaviors in the OFT. However, it did not affect the number of crossings.
Conclusion: Clofibrate as a PPAR-α receptors agonist may have antidepressant-like effect probably through increase in serotonin level and this central effect could not be attributed to generalized increase in locomotor activity of the animals.
Introduction
Major Depressive Disorder (MDD) is one of the most common illnesses with severe impacts on mortality and morbidity [1,2]. From the last several decades, many different medications based on monoamine neurotransmitters have been used [3,4]. Selective Serotonin (5-HT) Reuptake Inhibitors (SSRIs) such as Flouxetine (FLX) are the most frequently prescribed class of drugs for clinical depression. SSRIs are believed to exert their antidepressant ability blocking the reuptake of 5-HT at synaptic terminals, resulting in an elevation of extracellular 5-HT concentrations in the limbic regions that can act on various critical postsynaptic 5-HT receptors [5]. On the other hand, Tricyclic Antidepressants (TCAs) such as Desipramine (DMI) are selective for the Norepinephrine (NE) as compared to the serotonin transporter. The immediate effect of DMI is an inhibition of the NE Transporter (NET) resulting in an increase in NE [6,7]. Besides having a delay of 3–6 weeks for the current antidepressant drugs to achieve their antidepressant effect, various side effects have been reported [8]. Moreover, only about half of depressed patients taking monoamine-based antidepressants go into full remission [3]. The precise specification of the neural mechanisms underlying the effects of antidepressant drugs can identify novel candidates involved in the pathophysiology of depression and possible new targets for therapeutic intervention.
Peroxisome Proliferator-Activating Receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear receptor superfamily. Three molecular forms of PPAR have been identified, namely, PPAR-α, PPAR-β/δ, and PPAR-γ, and all involved in many different biological processes [9]. Peroxisome proliferator-activating receptor-alpha (PPAR-α ) is the predominant PPAR subtype highly expressed in the liver, heart, proximal tubules of the kidney cortex, the skeletal muscle, the intestinal mucosa, and in the brown adipose tissues that are metabolically very active [10]. PPAR-α is an important regulator of cellular fatty acid uptake and intracellular fatty acid transport, mitochondrial and peroxisomal fatty acid oxidation, ketogenesis, and gluconeogenesis [11,12]. It is well known that PPAR agonists protect against oxidative damage, inflammation, apoptosis in periphery, recent literature have described the neuroprotective role of PPAR agonists in the Central Nervous System (CNS) disorders [13]. PPAR-α exerted potential pharmacological properties on oxidative stress, inflammation, leukocyte-endothelium interactions, stem cells, and amyloid cascade [14-16].
Fibrates are a group of hypolipidemic agents which have been in clinical use for several decades in humans [17, 18]. It is well established that these agents act as synthetic agonists of PPAR-α [10]. Activation of PPAR by fibrates leads to increased hydrolysis of triglycerides, stimulation of cellular fatty acid uptake and conversion to acyl-CoA derivatives, decreased synthesis of triglycerides and fatty acids as well as Very-Low-Density Lipoprotein (VLDL) cholestrol, and finally increased peroxisomal and mitochondrial beta oxidation [17]. It has been shown that treatment of pigs with clofibrate (ethyl-α -p-chlorophenoxyisobutyrate), a PPAR-α agonist, stimulates mitochondrial and peroxisomal β-oxidation in the liver, muscle, and the kidney [19,20].
Thiazolidinediones (TZDs) are also a class of anti-diabetic drugs, which improve glucose and lipid metabolism reducing insulin resistance and used as an adjunctive therapy for treatment of Diabetes Mellitus (DM) [21, 22]. They act by binding to the peroxisome proliferator-activated receptor gamma (PPAR-γ). The PPAR-γ acts as a negative regulator of macrophage activation and PPAR-γ agonists inhibit the production of inflammatory cytokines in monocytes [23]. In cortical neuron-glia cocultures, PPAR-γ agonists inhibited both Lipopolysaccharide (LPS)-stimulated expression of Inducible Nitric Oxide Synthase (iNOS) in microglia and the Nitric Oxide (NO) release and COX-2 expression in neurons [24]. Similarly, PPAR-γ ligands reduced the iNOS expression and attenuated cell death in cerebellar granule cells [25]. It has been shown that PPAR-γ agonists improve different CNS dysfunctions [26,27]. Systemic treatment with PPAR-γ agonists, such as pioglitazone or troglitazone, improved the recovery from cerebral ischemia [28,29]. Moreover, antidepressant-like effect of pioglitazone in the Forced Swimming Test (FST) in mice has been shown and the role of PPAR-γ receptors stated [30].
Effects of fenofibrate, a PPAR-α agonist, on brain ischemia indicated that it modulates mechanisms involved in neurorepair and amyloid cascade. PPAR-α agonists could check the key points of a potential disease-modifying effect in stroke [31]. Also, fenofibrate was neuroprotective in Parkinson’s Disease (PD)-induced cognitive impairment in rats [32]. Oleoylethanolamide (OEA) is an endogenous lipid mediator acting as a PPAR-α agonist, facilitates memory consolidation through noradrenergic activation of the Basolateral complex of the amygdala (BLA) [33]. OEA administration could modulate the cognitive deficits [34].
Regarding the above information, we hypothesized that PPAR-α receptor agonist might attenuate the behavioral despair when exposed to an inescapable situation (forced swim) resemble to the PPAR-γ receptor agonists. The current study examined the effect of clofibrate, a PPAR-α receptor agonist, comparing two known antidepressant drugs, FLX and DMI on passive, immobility, and active behaviors, swimming and diving, in the modified rat FST.
Materials and Methods
Chemicals
Clofibrate (ethyl-a-p-chlorophenoxyisobutyrate), flouxetine and desiperamin hydrochloride were provided by Zahravi Pharmaceutical Co. (Tabriz, Iran).
Animals
Male Swiss Webster rats weighing 200-300 gram (Pasteur Institute, Tehran, Iran) were employed. The animals were housed in polycarbonate cages and maintained on a 12-hrs light: dark cycle in a temperature-controlled (22°C) condition. The animals had free access to food and water. Behavioral studies were carried out in the afternoon (12:00–4:00 PM). All procedures were carried out in accordance with the institutional guidelines for the animal care and use committee (NIH US publication, no. 23-86, revised 1985).
Experimental groups
A total of 45 rats were randomly assigned to 5 groups of 9. The drugs were administered in a sub-chronic manner. The drugs were administered three times at 2, 19 and 24 hrs. following the initial 15 min pre-test swim, prior to the 5 min swim test (the second day) [35]. All the drugs were freshly diluted in physiological saline except for clobibrate which was suspended in olive oil. One group received three distinct oral clofibrate administrations (300 mg/kg, p.o. [36,37], one group DMI (10 mg/kg, i.p.) [7], one group FLX (10 mg/kg, i.p.) [38], control groups received either saline (i.p.) or olive oil (p.o.) as vehicles.
Forced Swimming Test (FST)
In our study, behavioral evaluations were carried out in the afternoon (12:00-17:00) under low illumination. On day first (pre-test session), rats were placed individually in a Plexiglas cylinder, 46 cm height with a 21 cm internal diameter that was filled with water (25°C) to a depth of 30 cm. The animal was removed after 15 min, dried, and placed in its home cage. The pre-test facilitates the development of immobility during the test session and increases the sensitivity for detecting antidepressant behavioral effects [39]. The drugs were administered three times at 2, 19 and 24 hrs. Following the initial 15 min pre-test swim, prior to the 5 min swim test (the second day). Twenty-four hrs. After their first exposure (test session), the animals were replaced in the swim apparatus for 5 min, and the sessions were recorded manually using stopwatch for subsequent analysis. The 5 min swim test was used for analysis of the behavior. Water was changed between each swim session to prevent possible effects of an alarm substance released by rats during the swim session [40]. A time sampling technique was used to score several behaviors whereby the predominant behavior in at the end of each 5-sec period of the 300-sec test was recorded. The scorer would rate the dominant rat’s behavior at that time as one of the above four behaviors [41, 42]. The rat’s behavior was scored following the complete sampling method. The behaviors selected for scoring are swimming- the animal displays active swimming motions, more than necessary to maintain its head above water (usually horizontal) throughout the swim chamber, climbing- the rat makes active movements with its forepaws directed against the wall- and diving- the entire body is submerged beneath the water- behaviors as well as immobility- the animal floats in the water making only those movements necessary to keep its head above water [41,43,44].
Open Field Test (OFT)
The OFT was performed to evaluate general locomotor and rearing activity of the rats as described by [45]. The purpose of including this test was to assess the general activity of the animals after performing FST. The OFT [46] provides simultaneous measures of locomotion, exploration and anxiety. The number of line crosses and the frequency of rearing are usually used as measures of locomotor activity, but are also measures of exploration and anxiety. A high frequency of these behaviors indicates increased locomotion and exploration and/or a lower level of anxiety. The number of central square entries and the duration of time spent in the central square are measures of exploratory behavior and anxiety. A high frequency/duration of these behaviors indicates high exploratory behavior and low anxiety levels. The measurement parameters of this test include locomotor activity registered as the number of times the animal crosses squares and the rearing activity, which was registered as the number of times the animal stands upright on its hind legs. The central square is used because some strains have high locomotor activity and cross the lines of the test chamber many times during a test session. Also, the central square has sufficient space surrounding it to give meaning to the central location as being distinct from the outer locations [47]. Grooming behavior showed a moderate inverse correlation with the behavioral despair factor. On one hand, it suggests that grooming may be a good predictive behavior of impaired coping ability when the animal faces an uncontrollable stress situation, like FST. On the other hand, grooming that is considered a classical variable of OFT, appears distributed in both extracted factor and due to that, we considered it as an ambiguous variable, suggesting that the behavioral despair could have an anxiety component [48, 49]. The number of defection and urination as a level of anxiety were also recorded [50].
The open-field test chamber consisted of a white Plexiglas bin (56 × 38 × 30 cm) with the floor divided into 16 equal squares grids clearly drawn on the surface. An observer counted the number of line crosses during the 5 min test, recording the total line crosses at the end of each minute interval. Rats were gently placed on the center square and left to explore the floor for 5 min. Activities were manually recorded over a 5 min-period by a trained observer. The open field maze was cleaned between each rat using 70 % ethyl alcohol.
On the whole, the behaviors scored in our study included: Line crossing or locomotion (number of squares crossed): Frequency with which the rats crossed one of the grid lines with all four paws. Center square duration: Duration of time the rat spent in the central square; Rearing: Frequency with which the rats stood on their hind legs in the maze; Rearing against a wall: Frequency with which the rat stood on their hind legs against a wall of the open field; Grooming: Frequency and duration of time the animal spent licking or scratching itself while stationary; Center Square Entries: Frequency with which the rat crossed one of the red lines with all four paws into the central square. Defecation: number of fecal boli produced; Urination: number of puddles or streaks of urine [51].
Statistical Analysis
All the data were expressed as mean ± S.E.M. and analyzed using GraphPad Prism Statistics software package (version 6). Differences within experimental groups in immobility time and locomotors activity were analyzed by one-way Analysis Of Variance (ANOVA), whereas each of between groups differences (the interaction between clofibrate and the corresponding interventions) were analyzed by two-way ANOVA, both was followed by Tukey’s posttest. P < 0.05 was considered statistically significant in all experiments.
Results
Effect of clofibrate on FST and OFT
As can be seen in figure 1, three active behaviors, swimming, climbing and diving as well as the active behavior immobility were measured in the animals exposed to five min FST. Clofibrate (300 mg/kg, p.o.) increased swimming time in a significant manner (P < 0.001) and as a result, reduced the immobility period (P < 0.001). FLX (10 mg/kg, i.p.) also exerted a marked increase in the swimming measure and significantly decreased the immobility time (P < 0.001). On the other hand, the administration of DMI (10 mg/kg, i.p.) increased the climbing behavior; however, it did not affect the immobility measure (P < 0.001). Further, the drugs did not influence the diving behavior (Figure 1).
The results of OFT are demonstrated in Fig. 2. The results of the five behaviors were recorded in the animals during the five min session: number of crossings, number of rearing to the wall, number of rearing, number of entering to the central square, number of grooming. The results indicated that clofibrate (300 mg/kg, p.o.) markedly reduced the number of rearing (P < 0.05) and the number of rearing to the wall (P < 0.05). DMI (10 mg/kg, i.p.) exert a significant reduction on number of grooming (P < 0.01).
Figure 3 illustrates the number of defection and urination in OFT. There is not any significant difference between groups.
Discussion
In this study, we demonstrated that clofibrate, a PPAR-α receptors agonist, may attenuate the behavioral despair associated with forced swim exposure and also provided direct evidence further a role for PPAR-α receptor mediating this central effect was suggested for the first time. Sub-chronic clofibrate administration represented this protective effect reducing the immobility period and increasing the swimming period in the FST without affecting climbing and diving behaviors.
In the OFT, clofibrate also showed its activity by a marked reduction in number of the rearing and rearing to the wall which are mentioned as depression-like behaviors. In addition, only DMI reduced the number of grooming in this test and grooming behavior is a displacement response and is expected to be displayed in a novel environment [52]. Furthermore, this clofibrate effect could not be attributed to increase in the locomotor activity and it did not influence the generalized locomotor activity of the animals on the whole.
The pattern of behavioral effects produced by the antidepressant drugs suggested that enhancement of NE neurotransmission is related to climbing in the FST and that enhancement of 5-HT neurotransmission is related to swimming in the test [41]. Clofibrate in this study increased swimming comparable with FLX; therefore, the possibility that the serotonergic system is involved in the effect of clofibrate should not be ignored. Consistent with our experiment, it was found that antidepressant drugs such as DMI which inhibit NE reuptake and we used in our study, effectively reduced the immobility and selectively increased the climbing behavior without affecting the swimming [53,54], whereas FLX which we used in our study, works through the serotonin system, reduced the immobility and selectively increased the swimming, without affecting the climbing [55-57]. Climbing and diving behaviors were inversely correlated with the behavioral despair and therefore with the immobility [54].
According to previous studies, it is shown that PPAR-γ receptors are associated with the process of attenuation of depression [58]. Previously it was shown that PPAR-γ is expressed in restricted areas of the brain in rats such as the hippocampus, basal ganglia, frontal cortex, the hypothalamus and pituitary [59,60] which are known to be involved in depression [61,62]. The antidepressant- like effect of PPAR-γ agonists is demonstrated for the first time by pioglitazone, a PPAR-γ agonist, in a 55-year-old female who had severe unresponsive depression [63]. Furthermore, antidepressant-like effect of pioglitazone in the forced swimming test in mice has been shown and the role of PPAR-γ receptors stated [30]. NP031115, a novel TZDs, exerts antidepressant-like effect in mice, likely by inhibiting Glycogen Synthase Kinase-3 (GSK-3) and increasing PPAR-γ activity [58]. Rosiglitazone (RTZ), another TZDs and a PPAR-γ agonist, is also shown to have antidepressant-like effects in mice [64]. Previous studies have demonstrated that RTZ might improve learning and memory in both human and animal models [65-67].
Consistent with our study, swimming behavior was the most sensitive behavioral response to FLX [50,56]. The effect remains in agreement with the data published previously [68,69] which indicated that drugs affecting noradrenergic system modify rather climbing behavior, without any significant changes in the swimming. Indeed, the involvement of 5-HT vs. NE systems in action mechanism of SSRIs vs. NE Reuptake Inhibitors (NRIs) in the modified FST was shown by Page, et al. [53]. The modified FST differentiated swimming behavior, which was sensitive to SSRIs and 5-HT receptor agonists, and climbing behavior, which was sensitive to TCAs and drugs with selective effects on catecholamine transmission [41, 70]. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants [41]. DMI, increases rather climbing, while SSRIs – rather swimming behavior [71].
In agreement with a study in which DMI is well-known as potent and selective inhibitor of NE reuptake [72], we also observed increase in the climbing behavior. Porsolt et al suggested that DMI shortened immobility more than did two selective serotonin reuptake inhibitors, citaloperam and FLX [35]. However in our study, FLX and clofibrate were able to reduce the immobility time significantly.
Stretch attend postures are “risk-assessment” behaviors which indicate that the animal is hesitant to move from its present location to a new position [73] and thus a high frequency of these postures indicates a higher level of anxiety. Grooming behavior is a displacement response and is expected to be displayed in a novel environment [52]. Therefore, grooming behavior should decrease with repeated exposure to the testing apparatus. Defecation and urination are often used as measures of anxiety, however; the validity of defecation as a measure of anxiety has been questioned [74]. Hall [75] describes defecation and urination as indices of anxiety in rodents. He argues that the animal will have reduced locomotion in a novel environment, nevertheless; the autonomic nervous system will be activated which will increase defecation in this noxious arena. However, Bindra and Thompson [76] argue that there is no significant relation between fearfulness and urination and defecation as measured in the OFT. Repeated exposure to open field apparatus resulted in time-dependent changes in behavior [77]. At first, when the apparatus is novel to the animals, more fear-related behaviors (such as stretch attends and activity in the corners and walls of the open field) are displayed. However, with repeated trials, more exploration and locomotor activity (such as rearing and line crosses as well as more central square activity) is observed [78].
Effects of fenofibrate, a PPAR-α agonist, on acute and short-term consequences of brain ischemia indicated that fenofibrate modulates mechanisms involved in neurorepair and amyloid cascade. PPAR-α agonists could check the key points of a potential disease-modifying effect in stroke. Fenofibrate administration during the acute phase of experimentally induced brain ischemia has beneficial immediate and short-term neuroprotective effects [31]. Also, fenofibrate was neuroprotective in Parkinson’s disease-induced cognitive impairment in rats [32]. Acute and chronic PPAR-α agonists, including the clinically available fenofibrate, reduce nicotine-induced behavioral and Electroencephalographic (EEG) seizure expression and abolish nicotine-induced enhancement of Spontaneous Inhibitory Postsynaptic Currents (sIPSCs) in FCx pyramidal neurons [79].
OEA acting as a PPAR-α agonist, facilitates memory consolidation through noradrenergic activation of the basolateral amygdala (BLA) complex, a mechanism that is also critically involved in memory enhancement induced by emotional arousal [33]. OEA administration can modulate the cognitive deficits induced by 3, 4-Methylenedioxymethamphetamine (MDMA) in a Dopamine Transporter (DAT)-independent manner [34].
Conclusion
On the whole, one limitation of our study is that low doses of clofibrate were not examined in order to identify the initial dose which demonstrates this neuroprotective effect. As a consequence, further studies will be required to find a dose- response relationship for clofibrate. Moreover, selective PPAR-α antagonists could have been employed to clearly strengthen the involvement of PPAR-α receptors. Additionally, as we mentioned the possibility that clofibrate would be a serotonergic antidepressant which increases the active behavior, swimming, resemble to FLX [80], a 5-HT-related compound, a 5HT antagonist would be appropriate to determine if 5-HT depletion before clofibrate administration would block the anti-immmobility effect of clofibrate in the FST; for example using a tryptophan hydroxylase inhibitor, fenclonine, Para-Chlorophenylalanine (PCPA). On the other hand, other PPAR-α receptor agonists are suggested to be examined. To conclude, we have shown for the first time that clofibrate as a PPAR-α receptor agonist might have neuroprotective effect in behavioral despair.
- Schulz R, Drayer RA, Rollman BL. Depression as a risk factor for non-suicide mortality in the elderly. Biol Psychiatry. 2002; 52: 205-225. https://goo.gl/U36mwS
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007; 3: 137-158. https://goo.gl/3dRTWk
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci. 2006; 7: 137-151. https://goo.gl/FiS376
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, et al. Comparative benefits and harms of second-generation antidepressants: 405 background paper for the American College of Physicians. Ann Intern Med. 2008; 149: 734-750. https://goo.gl/ritx9u
- Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders- I. Basic pharmacology. J Psychopharmacol. 1998; 12: 5-20. https://goo.gl/BRVmxm
- Argenti D, D'Mello AP. Design of a desipramine dosing regimen for the rapid induction and maintenance of maximal cortical beta-adrenoceptor down-regulation. Neuropharmacology. 1994; 33: 1117-1124. https://goo.gl/oPo8ha
- Goodnough DB, Baker GB. 5-Hydroxytryptamine2 and beta-adrenergic receptor regulation in rat brain following chronic treatment with desipramine and fluoxetine alone and in combination. J Neurochem. 1994; 62: 2262-2268. https://goo.gl/yf4CMv
- Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci. 2002; 5: 1068-70. https://goo.gl/hvhnXR
- Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996; 12: 335-363. https://goo.gl/7CDnE6
- Beck F, Plummer S, Senior PV, Byrne S, Green S, Brammar WJ. The ontogeny of peroxisome-proliferator activated receptor gene expression in the mouse and rat. Proc Biol Sci. 1992; 22; 83-87. https://goo.gl/VQQ2e2
- Stott WT, Yano BL, Williams DM, Barnard SD, Hannah MA, Cieszlak FS, et al. Species-dependent induction of peroxisome proliferation by haloxyfop, an aryloxyphenoxy herbicide. Fundam Appl Toxicol. 1995; 28: 71-79. https://goo.gl/r8cKNN
- Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001; 7: 53-58. https://goo.gl/pFd2Kw
- Burston J and Kendall D. Peroxisome proliferator-activated receptors and inflammation, in endocannabinoids. Springer. 2012; 24: 221-233. https://goo.gl/foSQki
- Cimini A, Ceru MP. Emerging roles of peroxisome proliferator-activated receptors (PPARs) in the regulation of neural stem cells proliferation and differentiation. Stem Cell Rev. 2008; 4: 293-303. https://goo.gl/64ob5Q
- Cimini A, Benedetti E, D’Angelo B, Cristiano L, Falone S, Di Loreto S, et al. Neuronal response of peroxisomal and peroxisome-related proteins to chronic and acute A beta injury. Curr Alzheimer Res. 2009; 6: 238-251. https://goo.gl/ZeXXjy
- Gervois P, Fruchart JC, Staels B. Drug Insight: Mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat Clin Pract Endocrinol Metab. 2007; 3: 145-156. https://goo.gl/vdjmX2
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998; 98: 2088-2093. https://goo.gl/qJERgX
- Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-{alpha} activators: Clinical and Experimental Evidence. Arterioscler Thromb Vasc Biol. 2006; 26: 977-86. https://goo.gl/Cnh5m8
- Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-alpha and liver cancer: Where do we stand? J Mol Med (Berl). 2005; 83: 774-785. https://goo.gl/AhgH2N
- Yu XX, Odle J, Drackley JK. Differential induction of peroxisomal beta-oxidation enzymes by clofibric acid and aspirin in piglet tissues. Am J Physiol Regul Integr Comp Physiol. 2001; 281: 1553-1561. https://goo.gl/aajydV
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy. Update regarding the thiazolidinediones. Diabetologia. 2008; 51: 8-11. https://goo.gl/FbP21T
- McGuire DK, Inzucchi SE. New drugs for the treatment of diabetes mellitus: Part I: Thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008; 117: 440-449. https://goo.gl/D6PAEN
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998; 391: 79-82. https://goo.gl/KCRz5i
- Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: Associated with iNOS and COX-2. Brain Res. 2002; 941: 1-10. https://goo.gl/kaki3T
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000; 20: 6862-6867. https://goo.gl/nWRxJm
- Ramanan S, Zhao W, Riddle DR, Robbins ME. Role of PPARs in radiation-induced brain injury. PPAR Res. 2010; 2010: 234975. https://goo.gl/X4PJ2d
- Morgenweck J, Abdel-Aleem OS, McNamara KC, Donahue RR, Badr MZ, Taylor BK. Activation of peroxisome proliferator-activated receptor gamma in brain inhibits inflammatory pain, dorsal horn expression of Fos, and local edema. Neuropharmacology. 2010; 58: 337-45. https://goo.gl/GvPQd8
- Shiraz T, Inoue I, Araki N, Asano Y, Sawada M, Fuquay D, et al. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005; 36: 353-359. https://goo.gl/LkSeQy
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005; 130: 685-696. https://goo.gl/mbWsV8
- Salehi Sadaghiani M, Javadi-Paydar M, Gharedaghi MH, Yousefzadeh Fard Y, Dehpour AR. Antidepressant-like effect of pioglitazone in the forced swimming test in mice: The role of PPAR-gamma receptor and nitric oxide pathway. Behav Brain Res. 2011; 224: 336-343. https://goo.gl/iRQZMT
- Ouk T, Gautier S, Petrault M, Montaigne D, Marechal X, Masse I, et al. Effects of the PPAR-α agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cereb Blood Flow Metab. 2014; 34: 542-551. https://goo.gl/DdHZ6f
- Uppalapati D, Das NR, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS. Neuroprotective potential of peroxisome proliferator activated receptor-α agonist in cognitive impairment in Parkinson’s disease: Behavioral, Biochemical, and PBPK Profile. PPAR Research. 2014; 2014: 753587. https://goo.gl/sSWusn
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, et al. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci U S A. 2009; 106: 8027-8031. https://goo.gl/hNHZx3
- Plaza-Zabala A, Berrendero F, Suarez J, Bermudez-Silva FJ, Fernandez-Espejo E, Serrano A, et al. Effects of the endogenous PPAR-alpha agonist, oleoylethanolamide on MDMA-induced cognitive deficits in mice. Synapse. 2010; 64: 379-389. https://goo.gl/dcX5hY
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978; 47: 379-391. https://goo.gl/QVP1wL
- Seifert J, Mostecka H. Effect of clofibrate on DNA synthesis in rat liver and kidney. Arch Toxicol. 1987; 60:131-132. https://goo.gl/rPoZ26
- Baker VA, Harries HM, Waring JF, Duggan CM, Ni HA, Jolly RA, et al. Clofibrate-induced gene expression changes in rat liver: A cross-laboratory analysis using membrane cDNA arrays. Environ Health Perspect. 2004; 112: 428-438. https://goo.gl/iPcTyu
- Owolabi RA, Akanmu MA, Adeyemi OI. Effects of ketamine and N-methyl-D-aspartate on fluoxetine-induced antidepressant related behavior using the forced swimming test. Neurosci Lett. 2014; 566: 172-176. https://goo.gl/J8oWt1
- Borsini F, Lecci A, Sessarego A, Frassine R, Meli A. Discovery of antidepressant activity by forced swimming test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology. 1989; 97: 183-188. https://goo.gl/WSYJ34
- Abel EL, Bilitzke PJ. A possible alarm substance in the forced swimming test. Physiol Behav. 1990; 48: 233-239. https://goo.gl/gk2Y2Y
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl). 1995: 121: 66-72. https://goo.gl/zkjB8d
- Izadi Mobarakeh J, Fakhraei N, Abedini Sadr Z, Hamidipour A, Mostafavi E, Haji Hosseini R, et al. Effect of aqueous, ethanolic and acetonitrile Crocus sativus L. extracts on stress biomarkers in male rats. J Med Plants Res. 2013; 7: 3269-3279. https://goo.gl/Uq9JP7
- Porsolt RD. Animal Model of Depression. Biomedicine. 1979; 30: 139-140. https://goo.gl/4Zxbto
- Peng WH, Lo KL, Lee YH, Hung TH, Lin YC. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sciences. 2007; 81: 933-938. https://goo.gl/RkLSVH
- Wang SH, Zhang ZJ, Guo YJ, Zhou H, Teng GJ, Chen BA. Anhedonia and activity deficits in rats: Impact of poststroke depression. J Psychopharmacol. 2009; 23: 295-304. https://goo.gl/EZkcxo
- Walsh RN, Cummins RA. The open-field test: A critical review. Psychol Bull. 1976; 83: 482-504. https://goo.gl/ZUuRvJ
- Carrey N, McFadyen MP, Brown RE. Effects of subchronic methylphenidate administration on the locomotor and exploratory behaviour of prepubertal mice. J Child Adolesc Psychopharmacol. 2000; 10: 277-286. https://goo.gl/dM51dT
- Gorman JM. Comorbid depression and anxiety spectrum disorder. Depress Anxiety. 1996; 4: 160-168. https://goo.gl/vLfqck
- Ballenger JC. Clinical guideline for establishing remission in patients with depression and anxiety. J Clin Psychiatry. 1999; 60: 29-34. https://goo.gl/TMiqGo
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl). 2005; 182: 335-344. https://goo.gl/FeGDeE
- Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behavior Genetics. 1999; 26: 263-271. https://goo.gl/7Y6vxE
- Espejo EF. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav Brain Res. 1997; 87: 233-238. https://goo.gl/Zt1DPG
- Page ME, Dekte MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in rats forced swimming test. Psychopharmacol. 1999; 147:162-167. https://goo.gl/j3evKR
- Page ME, Brown K, Lucki I. Simultaneous analyses of the neurochemical and behavioural effects of the norepinephrine reuptake inhibitor reboxetine in a rat model of antidepressant action. Psychopharmacol. 2003; 165: 194-201. https://goo.gl/5bHvUG
- Lucki I, Singh A, Kreiss DS. Antidepressant-like behavioural effects of serotonin receptors agonists. Neurosci Biobehav Rev. 1994; 18:85-95. https://goo.gl/771fBU
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent Developments and future needs. Trends Pharmacol Sci. 2002; 23: 238-245. https://goo.gl/HvaQYf
- Thompson MR, Li KM, Clemens KJ, Gurtman CG, Hunt GE, Cornish JL, et al. Chronic fluoxetine treatment partly attenuates the long-term anxiety and depressive symptoms induced by NMDA (Ecstasy) in rats. Neuropsychopharmacol. 2004; 29: 694-704. https://goo.gl/AUq3YQ
- Rosa AO, Kaster MP, Binfare RW, Morales S, Martin-Aparicio E, Navarro-Rico ML, et al. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32: 1549-1556. https://goo.gl/WkGGC1
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004; 454: 131-145. https://goo.gl/BttUQN
- Cimini A, Benedetti E, Cristiano L, Sebastiani P, D’Amico MA, D’Angelo B, et al. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005; 130: 325-337. https://goo.gl/tjyXxj
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007; 155: 245-256. https://goo.gl/DqY7DZ
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: Bipolar disorder versus major depressive disorder. Bipolar Disord. 2008; 10: 1-37. https://goo.gl/FswtJY
- Kemp DE, Ismail-Beigi F, Calabrese JR. Antidepressant response associated with pioglitazone: Support for an overlapping pathophysiology between major depression and metabolic syndrome. Am J Psychiatry. 2009; 166: 619. https://goo.gl/Q3cDSC
- Eissa Ahmed AA, Al-Rasheed NM, Al Rasheed NM. Antidepressant-like effects of rosiglitazone, a PPAR gamma agonist, in the rat forced swim and mouse tail suspension tests. Behav Pharmacol. 2009; 20: 635-642. https://goo.gl/NuJii3
- Abbatecola AM, Lattanzio F, Molinari AM, Cioffi M, Mansi L, Rambaldi P, et al. Rosiglitazone and cognitive stability in older individuals with type 2 diabetes and mild cognitive impairment. Diabetes Care. 2010; 33: 1706-1711. https://goo.gl/T2iBeK
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: Results from a randomized, double-blind, placebo-controlledphase III study. Dement Geriatr Cogn Disord. 2010; 30: 131-146. https://goo.gl/LWiMbm
- Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res. 2011; 216: 255-261. https://goo.gl/a4rALo
- Hemby SE, Lucki I, Gatto G, Singh A, Thornley C, Matasi J, et al. Potential antidepressant effects of tropane compounds, selective for serotonin or dopamine transporters. J Pharmacol Exp Ther. 1997; 282: 727-733. https://goo.gl/mW3RRq
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacol. 2000; 22: 191-199. https://goo.gl/8ctUkW
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997; 8: 523-532. https://goo.gl/gskhwM
- Slater P, Blundell C. The effects of a permanent and selective depletion of brain catecholamines on the antinociceptive action of morphine. Naunyn Schmiedebergs Arch Pharmacol. 1978; 305: 227-232. https://goo.gl/wLtQeq
- Mayeda AR, Simon JR, Hingtgen JN, Hofstetter JR, Aprison MH. Activity-wheel stress and serotonergic hypersensitivity in rats. Pharmacol Biochem Behav. 1989; 33: 349-353. https://goo.gl/Fpp7Bq
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001; 25: 205-218. https://goo.gl/udkJUP
- Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990; 46: 321-340. https://goo.gl/6wx5UU
- Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934; 18: 382-403. https://goo.gl/ucqFB2
- Bindra D, Thompson WR. An evaluation of defecation and urination as measures of fearfulness. J Comp Physiol Psychol. 1953; 46: 43-45. https://goo.gl/SvFT64
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001; 25: 235-260. https://goo.gl/GbmrTD
- Bolivar V, Caldarone BJ, Reilly AA, Lorraine F. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behavior Genetics. 2000; 30: 285-293. https://goo.gl/yvtgne
- Puligheddu M, Pillolla G, Melis M, Lecca S, Marrosu F, De Montis MG, et al. PPAR-Alpha agonists as novel antiepileptic drugs: Preclinical Findings. Plos One. 2013; 8: 1-10. https://goo.gl/74Za6a
- Cryan JF, Lucki I. 5-HT4 receptors do not mediate the antidepressant-like behavioral effects of fluoxetine in a modified forced swim test. Eur J Pharmacol. 2000; 409: 295-299. https://goo.gl/C1x22H

Sign up for Article Alerts